

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50003896 (DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid::2-{(S)-4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid::2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-amino]-benzoylamino}-pentanedioic acid::2-{4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid::2-{4-[2-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid::CHEMBL170101
SMILES: Nc1nc2NCC(CCc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1
InChI Key: InChIKey=ZUQBAQVRAURMCL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50003896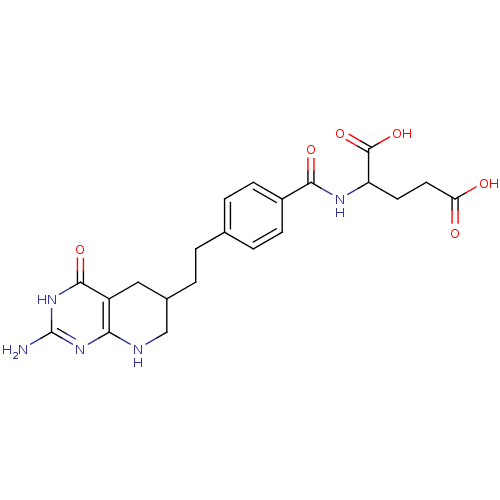 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GAR transformylase (Mus musculus) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for its inhibition of trifunctional glycinamide ribonucleotide formyltransferase (GARFT) isolated from murine L1210 leukemia cells | Bioorg Med Chem Lett 7: 453-456 (1997) Article DOI: 10.1016/S0960-894X(97)00041-3 BindingDB Entry DOI: 10.7270/Q2HT2PB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against beef liver dihydrofolate reductase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate Synthase (TS) (Lactobacillus casei) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Lactobacillus casei thymidylate synthetase | J Med Chem 28: 914-21 (1985) BindingDB Entry DOI: 10.7270/Q2CN74HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AICAR transformylase (Homo sapiens (Human)) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against MOLT-4 T-cell leukemia cell AICAR transformylase | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycinamide ribonucleotide formyltransferase (GARFTase) (Homo sapiens (Human)) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of hog liver GAR transformylase with (6R)-10-formyl-FH4 as cofactor | J Med Chem 35: 1399-410 (1992) BindingDB Entry DOI: 10.7270/Q20R9NBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||