

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50004016 CHEMBL138107::CHEMBL544159::N-(2-(2-(1-benzylpiperidin-4-yl)ethyl)-1,3-dioxoisoindolin-5-yl)benzamide::N-{2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl}-benzamide
SMILES: O=C(Nc1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1)c1ccccc1
InChI Key: InChIKey=JEXADSKXZBGTNK-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004016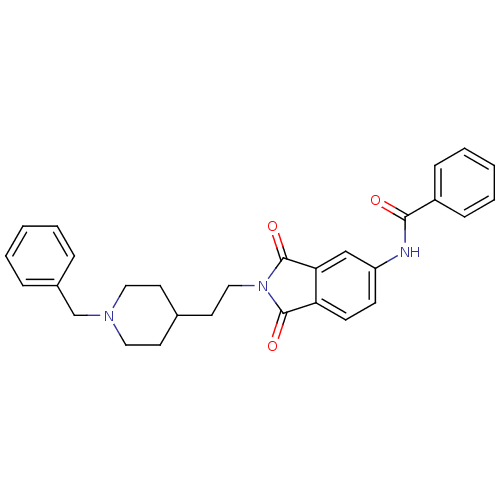 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004016 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against acetylcholinesterase | J Med Chem 38: 4821-9 (1996) BindingDB Entry DOI: 10.7270/Q2QC045T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004016 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE | Eur J Med Chem 45: 1167-72 (2010) Article DOI: 10.1016/j.ejmech.2009.12.038 BindingDB Entry DOI: 10.7270/Q25H7GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50004016 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against Butyrylcholinesterase obtained from rat plasma | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||