 Found 9 hits for monomerid = 50004152
Found 9 hits for monomerid = 50004152 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152
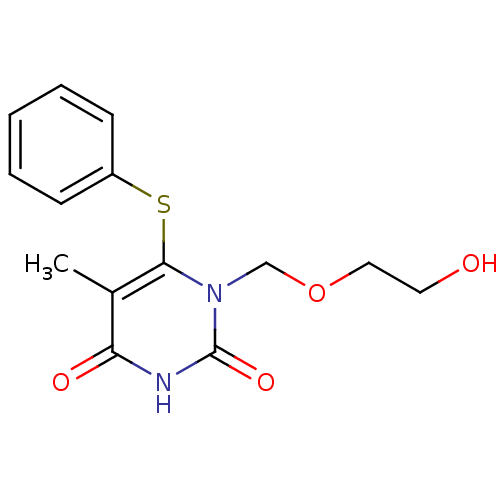
((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase from peripheral blood mononuclear cells. |
J Med Chem 34: 3305-9 (1991)
BindingDB Entry DOI: 10.7270/Q2DR2TFF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa University
Curated by ChEMBL
| Assay Description
Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rA)-oligo(dT) template primer |
J Med Chem 32: 2507-9 (1989)
BindingDB Entry DOI: 10.7270/Q2HH6J27 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells |
Bioorg Med Chem Lett 14: 3173-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.008
BindingDB Entry DOI: 10.7270/Q2862HNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa University
Curated by ChEMBL
| Assay Description
Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rC)-oligo(dG) template primer |
J Med Chem 32: 2507-9 (1989)
BindingDB Entry DOI: 10.7270/Q2HH6J27 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase at 37 degree centigrade |
J Med Chem 44: 145-54 (2001)
BindingDB Entry DOI: 10.7270/Q2VX0FRX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza
| Assay Description
Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity |
J Med Chem 47: 928-34 (2004)
Article DOI: 10.1021/jm0309856
BindingDB Entry DOI: 10.7270/Q26W988R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | >4.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells |
Bioorg Med Chem Lett 14: 3173-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.008
BindingDB Entry DOI: 10.7270/Q2862HNN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Human immunodeficiency virus type 1 reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50004152

((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-1 reverse transcriptase |
J Med Chem 39: 1589-600 (1996)
Article DOI: 10.1021/jm960056x
BindingDB Entry DOI: 10.7270/Q23X85QC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data