

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50004477 CCK7 analogue::CHEMBL269016::N-(1-Carbamoyl-2-phenyl-ethyl)-3-({2-[3-(1H-indol-3-yl)-2-(2-{2-[3-(4-sulfonyl-oxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-propionylamino]-hexanoyl}-methyl-amino)-succinamic acid
SMILES: CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O
InChI Key: InChIKey=YJRSAZMDFOJHLB-HECCNADXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50004477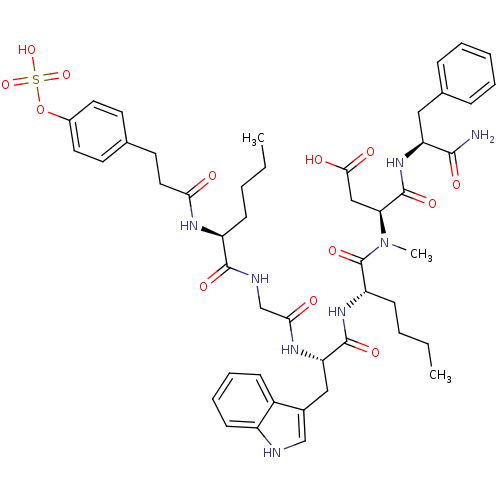 (CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Thermodynamic dissociation constant of compound for wild type E. coli dihydrofolate reductase | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin A receptor (Cavia porcellus) | BDBM50004477 (CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50004477 (CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50004477 (CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||