

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50004633 (S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]-5-(pyridin-2-ylcarbamoyl)-1,4-dihydro-pyridine-3-carboxylic acid ethyl ester::4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]-5-(pyridin-2-ylcarbamoyl)-1,4-dihydro-pyridine-3-carboxylic acid ethyl ester::4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]-5-(pyridin-2-ylcarbamoyl)-nicotinic acid ethyl ester::CHEMBL29067::UK-74505
SMILES: CCOC(=O)C1=C(N=C(C)C(C1c1ccccc1Cl)C(=O)Nc1ccccn1)c1ccc(cc1)-n1c(C)nc2cnccc12
InChI Key: InChIKey=RCODMGPJQVRMFZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet activating factor receptor (Cavia porcellus) | BDBM50004633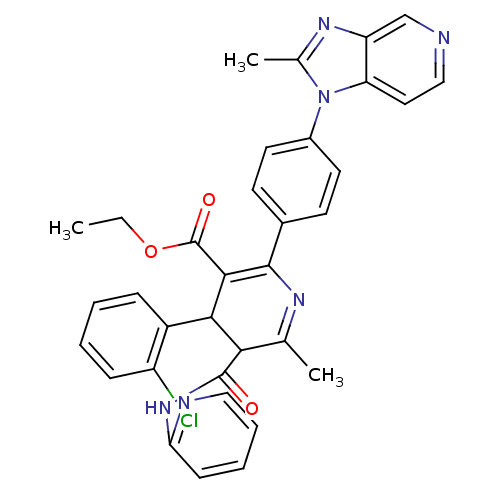 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-ligand from platelet activating factor (PAF) receptor in rabbit platelets | Bioorg Med Chem Lett 5: 1377-1382 (1995) Article DOI: 10.1016/0960-894X(95)00227-K BindingDB Entry DOI: 10.7270/Q2M045D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Cavia porcellus) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-leukotriene D4 (LTD4) from receptor in guinea pig lung membranes | Bioorg Med Chem Lett 5: 1377-1382 (1995) Article DOI: 10.1016/0960-894X(95)00227-K BindingDB Entry DOI: 10.7270/Q2M045D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated calcium channel (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis | J Med Chem 35: 3115-29 (1992) BindingDB Entry DOI: 10.7270/Q28W3DX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory effect on PAF induced platelets aggregation in rabbit | Bioorg Med Chem Lett 5: 3085-3090 (1995) Article DOI: 10.1016/0960-894X(95)00542-7 BindingDB Entry DOI: 10.7270/Q23F4PMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity for inhibition of [3H]PAF receptor binding to washed human platelet membranes. | Bioorg Med Chem Lett 2: 597-602 (1992) Article DOI: 10.1016/S0960-894X(01)81205-1 BindingDB Entry DOI: 10.7270/Q2CF9QK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory effect on PAF induced platelets aggregation in rabbit | Bioorg Med Chem Lett 5: 3085-3090 (1995) Article DOI: 10.1016/0960-894X(95)00542-7 BindingDB Entry DOI: 10.7270/Q23F4PMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory effect on PAF induced platelets aggregation in rabbit | Bioorg Med Chem Lett 5: 3085-3090 (1995) Article DOI: 10.1016/0960-894X(95)00542-7 BindingDB Entry DOI: 10.7270/Q23F4PMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated calcium channel (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [3H]nitrendipine from L-type calcium channel of bovine frontal cortex membranes. | J Med Chem 35: 3115-29 (1992) BindingDB Entry DOI: 10.7270/Q28W3DX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Cavia porcellus) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of PAF-induced aggregation of rabbit washed platelets. | J Med Chem 35: 3115-29 (1992) BindingDB Entry DOI: 10.7270/Q28W3DX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Cavia porcellus) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Cavia porcellus) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Antagonistic activity was tested against Platelet-Activating Factor-induced aggregation of rabbit washed platelets. | J Med Chem 38: 3514-23 (1995) BindingDB Entry DOI: 10.7270/Q2PK0GSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated calcium channel (Homo sapiens (Human)) | BDBM50004633 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Displacement of [3H]-diltiazem from L-type calcium channel of bovine frontal cortex membranes. | J Med Chem 35: 3115-29 (1992) BindingDB Entry DOI: 10.7270/Q28W3DX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||