

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50004783 3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-ethyl)-3,7,12,17-tetramethyl-22,24-dihydro-porphin-2-yl]-propionic acid::CHEMBL408667
SMILES: Cc1c(CCC(O)=O)c2cc3nc(cc4[nH]c(cc5[nH]c(cc1n2)c(C)c5C(COC(=O)C1=CCC1)OC(=O)C1=CCC1)c(C)c4C(COC(=O)C1=CCC1)OC(=O)C1=CCC1)c(C)c3CCC(O)=O
InChI Key: InChIKey=XWLXUFRSYMFDLQ-WNHXZLMMSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 2 pol protein (Human immunodeficiency virus 2) | BDBM50004783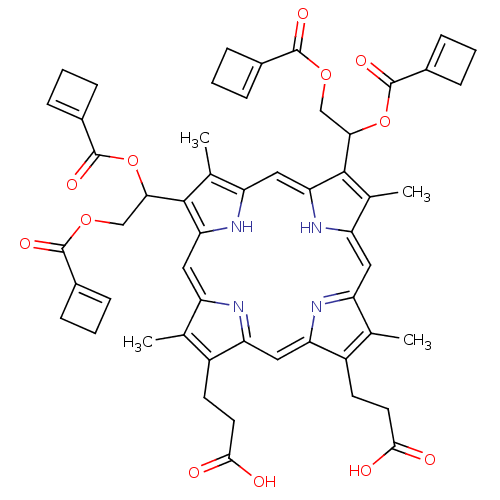 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-2 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 5%DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human rhinovirus A protease (Human rhinovirus B) | BDBM50004783 (3-[18-(2-Carboxy-ethyl)-8,13-bis-(1,2-diacetoxy-et...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease in the absence of DMSO | J Med Chem 35: 3426-8 (1992) BindingDB Entry DOI: 10.7270/Q27W6CTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||