

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50005701 CHEMBL337283::N*1*-[1-Benzyl-3-(2-tert-butylcarbamoyl-pyrrolidin-1-yl)-2-hydroxy-3-oxo-propyl]-2-[(quinoline-2-carbonyl)-amino]-succinamide
SMILES: CC(C)(C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1
InChI Key: InChIKey=JVBMLUUKYDVROE-OBXRUURASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50005701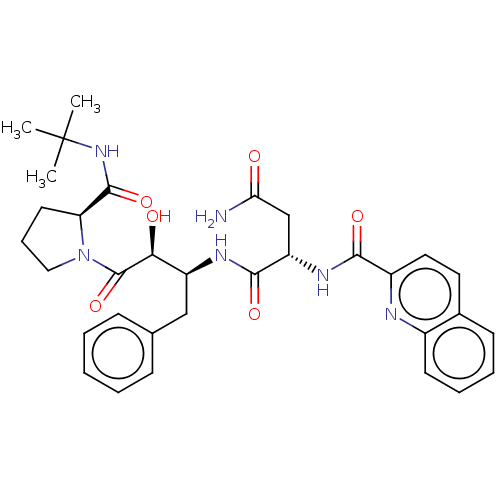 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50005701 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme (by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50005701 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Inhibitory potency against HIV-1 protease | J Med Chem 36: 4152-60 (1994) BindingDB Entry DOI: 10.7270/Q2PK0HCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human rhinovirus A protease (Human rhinovirus B) | BDBM50005701 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 145 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antiviral activity of the compound against HTLV-1 RF infected MT-2 cells | Bioorg Med Chem Lett 6: 435-438 (1996) Article DOI: 10.1016/0960-894X(96)00034-0 BindingDB Entry DOI: 10.7270/Q2W095X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50005701 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 435-438 (1996) Article DOI: 10.1016/0960-894X(96)00034-0 BindingDB Entry DOI: 10.7270/Q2W095X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||