

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50005860 2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[2,3-d]pyrimidin-6-ylmethyl)-amino]-benzoylamino}-4-(ethoxy-hydroxy-phosphoryl)-butyric acid::CHEMBL41459
SMILES: CCOP(O)(=O)CCC(NC(=O)c1ccc(NCC2CNc3nc(N)[nH]c(=O)c3C2)cc1)C(O)=O
InChI Key: InChIKey=GPUSXKHIVFTONZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Folylpoly-gamma-glutamate synthetase (Mus musculus) | BDBM50005860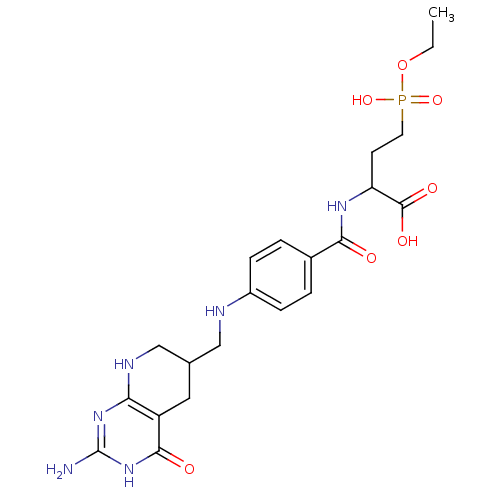 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005860 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of human thymidylate synthase (TS) | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50005860 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of human dihydrofolate reductase (DHFR) | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||