

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50007104 CHEMBL3237572::US10112928, Compound I-17
SMILES: CC(C)C[C@H](CN)Nc1cc(Nc2cccc(n2)C(C)(C)C)c(nn1)C(N)=O
InChI Key: InChIKey=MXHGIPNEGMISFM-CYBMUJFWSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007104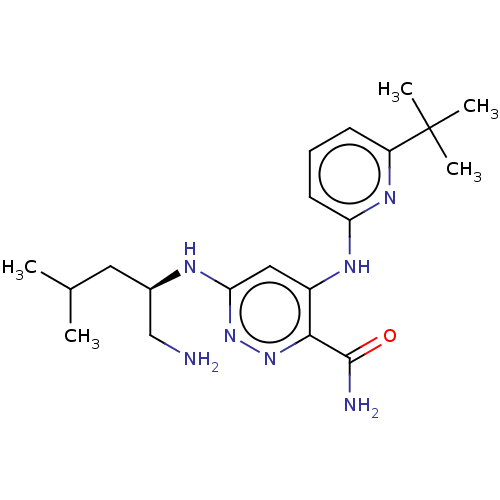 (CHEMBL3237572 | US10112928, Compound I-17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human SYK (360 to 635) using biotin-EPEGDYEEVLE as substrate preincubated for 10 mins followed by substrate/ATP/[33Pgamma]ATP addition ... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SYK RPA purified, truncated construct of Spleen Tyrosine Kinase (aa 360-635) (Homo sapiens (Human)) | BDBM50007104 (CHEMBL3237572 | US10112928, Compound I-17) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK kinase assay is a standard kinase assay adapted to a 96 well plate format. This assay is performed in 96-well format for IC50 determination with ... | US Patent US10112928 (2018) BindingDB Entry DOI: 10.7270/Q2Z03B68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50007104 (CHEMBL3237572 | US10112928, Compound I-17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by patch clamp method | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50007104 (CHEMBL3237572 | US10112928, Compound I-17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of SYK in human whole blood assessed as anti-human IgM-induced CD69 protein expression preincubated for 30 mins followed by anti-human IgM... | J Med Chem 57: 2683-91 (2014) Article DOI: 10.1021/jm401982j BindingDB Entry DOI: 10.7270/Q2HT2QTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||