

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50010135 CHEMBL548400
SMILES: NC(=N)c1ccc(OCc2cccc(COc3ccc(cc3I)C(N)=N)c2)c(I)c1
InChI Key: InChIKey=QQTXOFVYQMNURU-UHFFFAOYSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glandular kallikrein (Sus scrofa) | BDBM50010135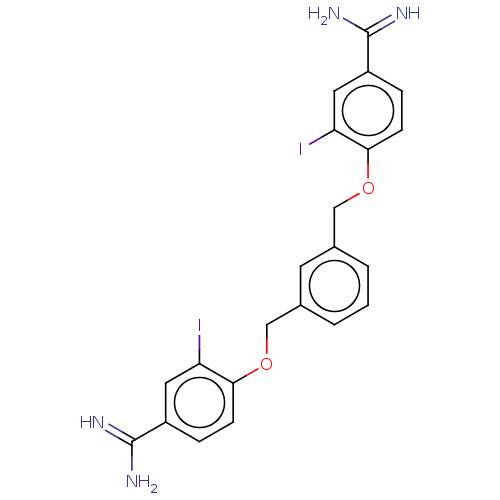 (CHEMBL548400) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic kallikrein using BPPANA as substrate after 15 to 180 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombin (Bos taurus (Bovine)) | BDBM50010135 (CHEMBL548400) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of amidase activity of bovine thrombin using BANA as substrate after 15 to 40 mins | J Med Chem 19: 634-9 (1976) BindingDB Entry DOI: 10.7270/Q2KD20FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Sus scrofa) | BDBM50010135 (CHEMBL548400) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of boar spermatozoa acrosin using BzArgOEt as substrate by Dixon plot analysis | J Med Chem 21: 1132-6 (1979) BindingDB Entry DOI: 10.7270/Q2TT4SGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||