

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50012171 CHEMBL3264474
SMILES: O=C([C@@H]1COc2cc(Oc3ccccc3)ccc2C1)c1ncc(o1)-c1ccccn1
InChI Key: InChIKey=KTRKLISDHZZCIZ-KRWDZBQOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM50012171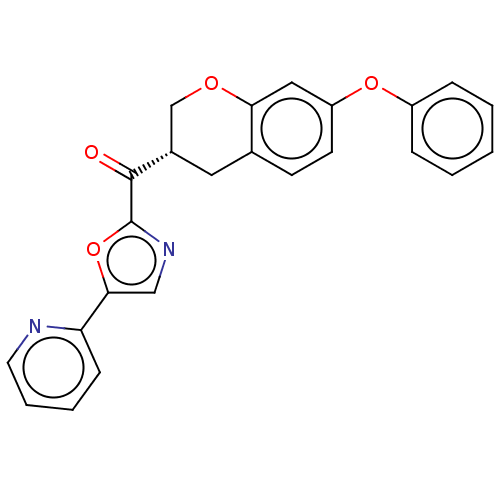 (CHEMBL3264474) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of rat recombinant FAAH expressed in Escherichia coli using [14C]oleamide as substrate assessed as oleic acid formation by Dixon plot anal... | Bioorg Med Chem 22: 2763-70 (2014) Article DOI: 10.1016/j.bmc.2014.03.013 BindingDB Entry DOI: 10.7270/Q2X92CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Abhydrolase Domain-Containing Protein 6 (Mus musculus (mouse)) | BDBM50012171 (CHEMBL3264474) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse brain ABHD6 incubated for 10 mins prior to rhodamine-tagged fluorophosphonate addition measured after 10 mins by SDS-PAGE | Bioorg Med Chem 22: 2763-70 (2014) Article DOI: 10.1016/j.bmc.2014.03.013 BindingDB Entry DOI: 10.7270/Q2X92CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride Lipase (MGL) (Mus musculus (mouse)) | BDBM50012171 (CHEMBL3264474) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse brain MAGL incubated for 10 mins prior to rhodamine-tagged fluorophosphonate addition measured after 10 mins by SDS-PAGE | Bioorg Med Chem 22: 2763-70 (2014) Article DOI: 10.1016/j.bmc.2014.03.013 BindingDB Entry DOI: 10.7270/Q2X92CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anandamide amidohydrolase (Mus musculus (mouse)) | BDBM50012171 (CHEMBL3264474) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse brain FAAH incubated for 10 mins prior to rhodamine-tagged fluorophosphonate addition measured after 10 mins by SDS-PAGE | Bioorg Med Chem 22: 2763-70 (2014) Article DOI: 10.1016/j.bmc.2014.03.013 BindingDB Entry DOI: 10.7270/Q2X92CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KIAA1363 (Mus musculus (mouse)) | BDBM50012171 (CHEMBL3264474) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse brain KIAA1363 incubated for 10 mins prior to rhodamine-tagged fluorophosphonate addition measured after 10 mins by SDS-PAGE | Bioorg Med Chem 22: 2763-70 (2014) Article DOI: 10.1016/j.bmc.2014.03.013 BindingDB Entry DOI: 10.7270/Q2X92CVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||