

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50013712 2-aminopyridin::2-aminopyridine::CHEMBL21619::Pyridin-2-ylamine
SMILES: Nc1ccccn1
InChI Key: InChIKey=ICSNLGPSRYBMBD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil cytosol factor 1 (Homo sapiens) | BDBM50013712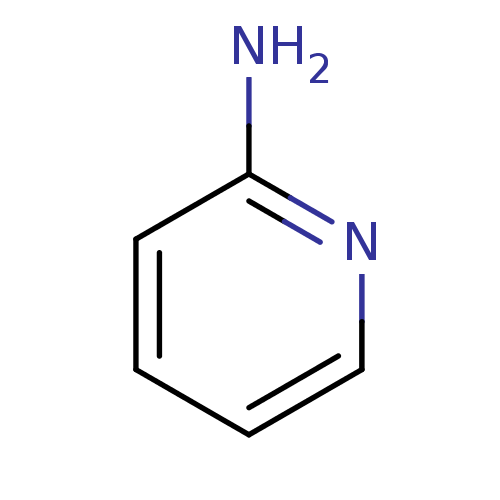 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Covalent inhibition of recombinant human His-tagged p47phox SH3A-B domain (151 to 285 residues) expressed in Escherichia coli BL21 (DE3) cells intera... | J Med Chem 63: 1156-1177 (2020) Article DOI: 10.1021/acs.jmedchem.9b01492 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against inducible nitric oxide synthase (iNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TEC (Mus musculus) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >4.00E+6 | n/a | n/a | n/a | n/a | n/a |
The University of Adelaide Curated by ChEMBL | Assay Description Displacement of PRP-1 peptide from mouse Tec kinase SH3 domain by fluorescence polarization | J Med Chem 47: 5405-17 (2004) Article DOI: 10.1021/jm049533z BindingDB Entry DOI: 10.7270/Q26M369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of human nNOS by hemoglobin capture assay | J Med Chem 52: 4533-7 (2009) Article DOI: 10.1021/jm900380j BindingDB Entry DOI: 10.7270/Q2TQ61G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >1.00E+9 | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human HSP90alpha assessed as 2D1H-15N chemical shift perturbation by NMR spectroscopy | Bioorg Med Chem Lett 21: 5778-83 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.08.001 BindingDB Entry DOI: 10.7270/Q2QJ7HQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase type 2-alpha (Homo sapiens (Human)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Research Limited Curated by ChEMBL | Assay Description Inhibitory activity (IC50) against human phosphatidylinositol 4-kinase at the ATP binding site | J Med Chem 33: 2073-80 (1990) BindingDB Entry DOI: 10.7270/Q25T3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against neuronal nitric oxide synthase | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50013712 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of wild type rat nNOS by hemoglobin capture assay | J Med Chem 52: 4533-7 (2009) Article DOI: 10.1021/jm900380j BindingDB Entry DOI: 10.7270/Q2TQ61G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||