

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50014716 CHEMBL3262025::US10759791, Compound 8S::US9951014, Name 8S
SMILES: Cc1cc(N)nc(CCc2cncc(c2)[C@@H](CN)Cc2cc(C)cc(N)n2)c1
InChI Key: InChIKey=HKLFDCGBVFYQPQ-QGZVFWFLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50014716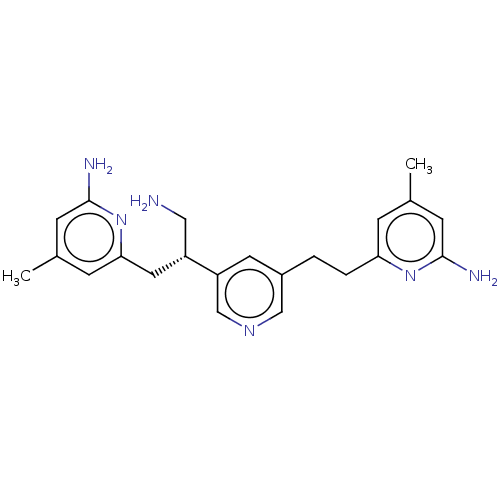 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... | J Med Chem 52: 379-88 (2009) BindingDB Entry DOI: 10.7270/Q21N83G1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of purified rat nNOS assessed as inhibition of nitric oxide-mediated oxidation of hemoglobin-A by hemoglobin capture assay | J Med Chem 57: 4382-96 (2014) Article DOI: 10.1021/jm5004182 BindingDB Entry DOI: 10.7270/Q21R6S26 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric Oxide Synthase, inducible (Mus musculus (mouse)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of purified mouse iNOS assessed as inhibition of nitric oxide-mediated oxidation of hemoglobin-A by hemoglobin capture assay | J Med Chem 57: 4382-96 (2014) Article DOI: 10.1021/jm5004182 BindingDB Entry DOI: 10.7270/Q21R6S26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... | J Med Chem 52: 379-88 (2009) BindingDB Entry DOI: 10.7270/Q21N83G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric Oxide Synthase, endothelial (Bos taurus (bovine)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of purified bovine eNOS assessed as inhibition of nitric oxide-mediated oxidation of hemoglobin-A by hemoglobin capture assay | J Med Chem 57: 4382-96 (2014) Article DOI: 10.1021/jm5004182 BindingDB Entry DOI: 10.7270/Q21R6S26 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... | J Med Chem 52: 379-88 (2009) BindingDB Entry DOI: 10.7270/Q21N83G1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric-oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50014716 (CHEMBL3262025 | US10759791, Compound 8S | US995101...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||