

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50014843 2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-5-yl)-propyl]-formyl-amino}-benzoylamino)-pentanedioic acid::CHEMBL152172
SMILES: Nc1nc(N)c(CCCN(C=O)c2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(=O)[nH]1
InChI Key: InChIKey=KOOBXXUWGKUQCV-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AICAR transformylase (Mus musculus) | BDBM50014843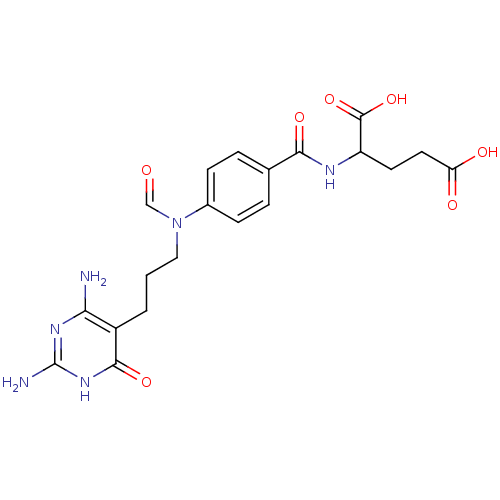 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AICAR transformylase (Mus musculus) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Hexaglutamyl homologue inhibition activity against the AICAR formyltransferase was determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase/GAR transformylase/AICAR transformylase (Homo sapiens (Human)) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycinamide ribonucleotide formyltransferase (GARFTase) (Homo sapiens (Human)) | BDBM50014843 (2-(4-{[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against L cell | J Med Chem 33: 561-7 (1990) BindingDB Entry DOI: 10.7270/Q2XW4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||