

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: ONC(=O)\C=C\c1ccccc1
InChI Key: InChIKey=UVDDFTZLVFIQFL-VOTSOKGWSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50015088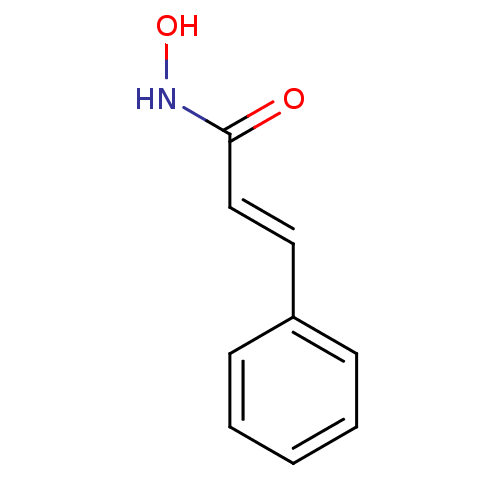 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Logarithmic value of inhibitory concentration against 5-lipoxygenase in rat basophilic leukemia cells (RBL-1) | J Med Chem 33: 992-8 (1990) BindingDB Entry DOI: 10.7270/Q2WW7GNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of HDAC4 (unknown origin) assessed as fluorescence intensity measured after 60 mins incubation at room temperature by trypsin-free microfl... | J Med Chem 56: 1772-6 (2013) Article DOI: 10.1021/jm301355j BindingDB Entry DOI: 10.7270/Q2XK8GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 457 | n/a | n/a | n/a | n/a | n/a | 25 |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of HDAC2 (unknown origin) assessed as fluorescence intensity measured after 60 mins incubation at room temperature by trypsin-free microfl... | J Med Chem 56: 1772-6 (2013) Article DOI: 10.1021/jm301355j BindingDB Entry DOI: 10.7270/Q2XK8GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | 25 |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) assessed as fluorescence intensity measured after 60 mins incubation at room temperature by trypsin-free microfl... | J Med Chem 56: 1772-6 (2013) Article DOI: 10.1021/jm301355j BindingDB Entry DOI: 10.7270/Q2XK8GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | 25 |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) assessed as fluorescence intensity measured after 60 mins incubation at room temperature by trypsin-free microfl... | J Med Chem 56: 1772-6 (2013) Article DOI: 10.1021/jm301355j BindingDB Entry DOI: 10.7270/Q2XK8GWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against 5-lipoxygenase in rat basophilic leukemia cells(RBL-1) | J Med Chem 33: 992-8 (1990) BindingDB Entry DOI: 10.7270/Q2WW7GNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50015088 ((E)-N-Hydroxy-3-phenyl-acrylamide | (E)-N-Hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against RBL-1 5-LO | J Med Chem 30: 574-80 (1987) BindingDB Entry DOI: 10.7270/Q2QN67BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||