

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50017478 Amastatin::CHEMBL28650::Leu[1psi,CHOHCONH]ValValAsp::N-[(2S,3R)-3-amino-2-hydroxy-5-methylhexanoyl]-L-valyl-L-valyl-L-aspartic acid
SMILES: CC(C)C[C@@H](N)[C@H](O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O
InChI Key: InChIKey=QFAADIRHLBXJJS-ZAZJUGBXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478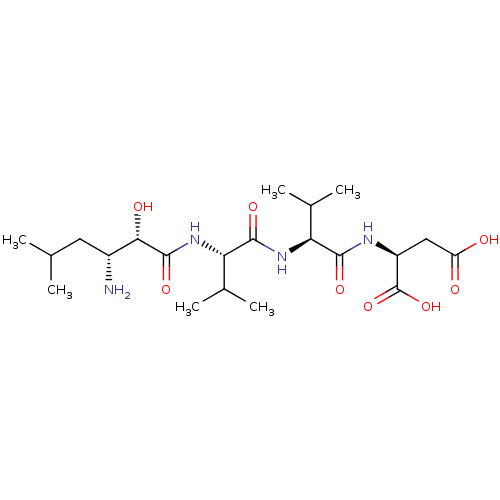 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Aminopeptidase M from porcine kidney was determined and Ki was reported which is obta... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of aminopeptidase N (APN) | J Med Chem 37: 1339-46 (1994) BindingDB Entry DOI: 10.7270/Q2K074ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and Ki* was reported which is obtained by the equatio... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase A (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of aminopeptidase A (APA) | J Med Chem 37: 1339-46 (1994) BindingDB Entry DOI: 10.7270/Q2K074ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase (Homo sapiens (Human)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of aminopeptidase M was determined and the Ki was reported which is = k2/k1 | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50017478 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect of inhibitor structure on the slow binding inhibition of Leucine aminopeptidase was determined and Ki* was reported which is obtained by the e... | J Med Chem 27: 417-22 (1984) BindingDB Entry DOI: 10.7270/Q280535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||