

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50018008 CHEMBL3289671::US9238653, Table 5, Compound 43
SMILES: Cc1cc(Nc2cc(F)c(c(F)c2)C(F)(F)F)n2nc(nc2n1)C(C)(F)F
InChI Key: InChIKey=KKCBTRGIGVWQFK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum (isolate 3D7)) | BDBM50018008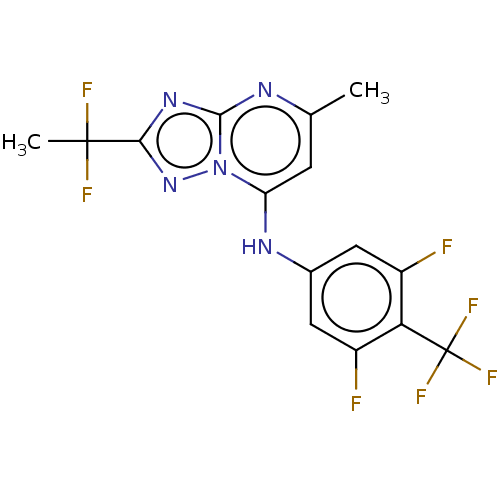 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; MMV MEDICINES FOR MALARIA VENTURE; UNIVERSITY OF WASHINGTON US Patent | Assay Description For studying inhibition of Plasmodium or human DHODH enzyme, two assays that are in routine use are described, for example, in Baldwin, et al. (2002)... | US Patent US9238653 (2016) BindingDB Entry DOI: 10.7270/Q2D79954 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (Homo sapiens (Human)) | BDBM50018008 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center at Dallas Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human DHODH (amino acid residues 30 to 396) expressed in Escherichia coli BL21 cells assessed as orotic acid pro... | J Med Chem 57: 5381-94 (2014) Article DOI: 10.1021/jm500481t BindingDB Entry DOI: 10.7270/Q2WM1FZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (Mus musculus) | BDBM50018008 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center at Dallas Curated by ChEMBL | Assay Description Inhibition of mouse DHODH (amino acid residues 30 to 396) expressed in Escherichia coli BL21 cells assessed as orotic acid production using dihydroor... | J Med Chem 57: 5381-94 (2014) Article DOI: 10.1021/jm500481t BindingDB Entry DOI: 10.7270/Q2WM1FZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate Dehydrogenase (DHODH) (Rattus norvegicus (rat)) | BDBM50018008 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center at Dallas Curated by ChEMBL | Assay Description Binding affinity to rat DHODH by isothermal titration calorimetry | J Med Chem 57: 5381-94 (2014) Article DOI: 10.1021/jm500481t BindingDB Entry DOI: 10.7270/Q2WM1FZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate Dehydrogenase (DHODH) (Rattus norvegicus (rat)) | BDBM50018008 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center at Dallas Curated by ChEMBL | Assay Description Inhibition of rat DHODH (amino acid residues 30 to 396) expressed in Escherichia coli BL21 cells assessed as orotic acid production using dihydroorot... | J Med Chem 57: 5381-94 (2014) Article DOI: 10.1021/jm500481t BindingDB Entry DOI: 10.7270/Q2WM1FZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (Homo sapiens (Human)) | BDBM50018008 (CHEMBL3289671 | US9238653, Table 5, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Medical Center at Dallas Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human DHODH (amino acid residues 30 to 396) expressed in Escherichia coli BL21 cells assessed as orotic acid pro... | J Med Chem 57: 5381-94 (2014) Article DOI: 10.1021/jm500481t BindingDB Entry DOI: 10.7270/Q2WM1FZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||