

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50018556 (+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione::(+/-)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione::(-)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione::1'-Benzyl-3-phenyl-[3,4']bipiperidinyl-2,6-dione::CHEMBL10272::Dexetimide1'-Benzyl-3-phenyl-[3,4']bipiperidinyl-2,6-dione
SMILES: O=C1CCC(C2CCN(Cc3ccccc3)CC2)(C(=O)N1)c1ccccc1
InChI Key: InChIKey=LQQIVYSCPWCSSD-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| chemokine (C-X-C motif) receptor 3 (Homo sapiens (Human)) | BDBM50018556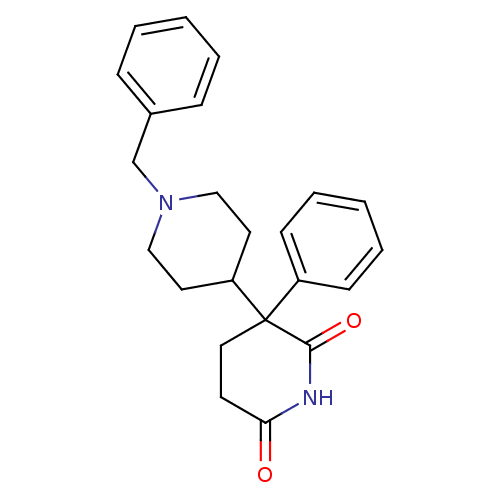 ((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson PRD Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 expressed in CHO cells assessed as inhibition of ITAC-stimulated [35S]GTPgammaS binding pretreated 30 mins before ... | Bioorg Med Chem Lett 18: 5819-23 (2009) Article DOI: 10.1016/j.bmcl.2008.07.115 BindingDB Entry DOI: 10.7270/Q2RV0NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50018556 ((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...) | UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson PRD Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M1 receptor | Bioorg Med Chem Lett 18: 5819-23 (2009) Article DOI: 10.1016/j.bmcl.2008.07.115 BindingDB Entry DOI: 10.7270/Q2RV0NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Mus musculus) | BDBM50018556 ((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions Curated by ChEMBL | Assay Description Ability (10 ug/kg) to inhibit binding of [125I]iododexetimide to muscarinic receptor in mice | J Med Chem 32: 1057-62 (1989) BindingDB Entry DOI: 10.7270/Q29S1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50018556 ((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson PRD Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M3 receptor | Bioorg Med Chem Lett 18: 5819-23 (2009) Article DOI: 10.1016/j.bmcl.2008.07.115 BindingDB Entry DOI: 10.7270/Q2RV0NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (RAT) | BDBM50018556 ((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins Medical Institutions Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from rat brain homogenate | J Med Chem 32: 1057-62 (1989) BindingDB Entry DOI: 10.7270/Q29S1T87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50018556 ((+)-1'-benzyl-3-phenyl-3,4'-bipiperidine-2,6-dione...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson PRD Curated by ChEMBL | Assay Description Binding affinity to human muscarinic M2 receptor | Bioorg Med Chem Lett 18: 5819-23 (2009) Article DOI: 10.1016/j.bmcl.2008.07.115 BindingDB Entry DOI: 10.7270/Q2RV0NQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||