

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50019292 CHEMBL3289393
SMILES: Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO[P@@](O)(=S)OP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O
InChI Key: InChIKey=LINQALDDRNLFQP-KAUSDDJZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019292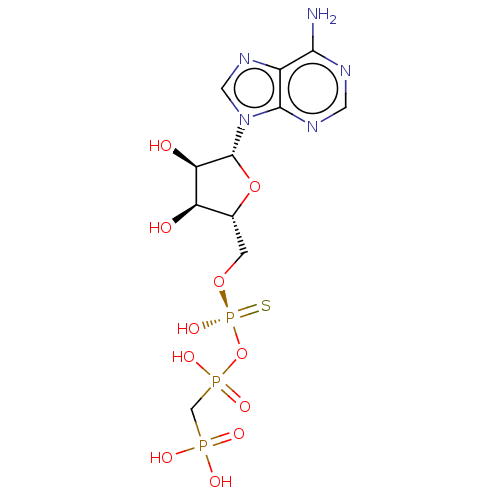 (CHEMBL3289393) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y11 (Homo sapiens (Human)) | BDBM50019292 (CHEMBL3289393) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y11 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019292 (CHEMBL3289393) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||