

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50020168 1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzylamino)hexylamino]ethyldisulfanyl}ethyl)-1,6-hexanediamine::CHEMBL19060::N-(2-Methoxy-benzyl)-N'-(2-{2-[6-(2-methoxy-benzylamino)-hexylamino]-ethyldisulfanyl}-ethyl)-hexane-1,6-diamine::N-(2-Methoxy-benzyl)-N'-(2-{2-[6-(2-methoxy-benzylamino)-hexylamino]-ethyldisulfanyl}-ethyl)-hexane-1,6-diamine (BHC, Benextramine)::N-(2-Methoxy-benzyl)-N'-(2-{2-[6-(2-methoxy-benzylamino)-hexylamino]-ethyldisulfanyl}-ethyl)-hexane-1,6-diamine .4HCl::N1-(2-methoxybenzyl)-N6-(2-(2-(2-(6-(2-methoxybenzylamino)hexylamino)ethyl)disulfanyl)ethyl)hexane-1,6-diamine
SMILES: COc1ccccc1CNCCCCCCNCCSSCCNCCCCCCNCc1ccccc1OC
InChI Key: InChIKey=IIWOUNLDWKZMQI-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50020168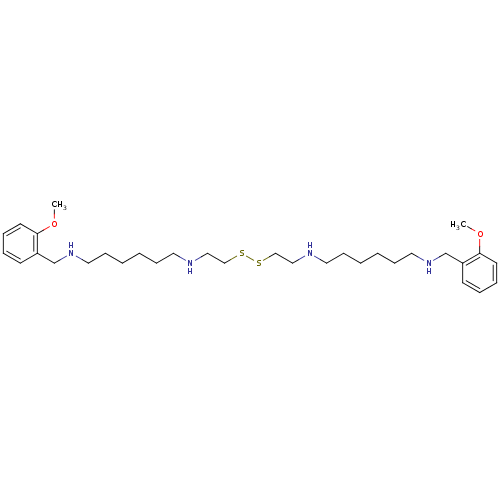 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to NPY1 receptor | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 ( NPY Y5) (Homo sapiens (Human)) | BDBM50020168 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to NPY5 receptor | Bioorg Med Chem Lett 21: 5436-41 (2011) Article DOI: 10.1016/j.bmcl.2011.06.124 BindingDB Entry DOI: 10.7270/Q2RN387K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020168 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human AchE | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha (Rattus norvegicus (rat)-Rattus norvegicus (Rat)-RA...) | BDBM50020168 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against alpha adrenergic receptor in rat | J Med Chem 28: 1643-7 (1985) BindingDB Entry DOI: 10.7270/Q23T9KF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020168 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) of human erythrocytes | J Med Chem 41: 4186-9 (1998) Checked by Author Article DOI: 10.1021/jm9810452 BindingDB Entry DOI: 10.7270/Q2HH6KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha-2 (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50020168 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Alpha-2 adrenergic receptor blocking activity by antagonism of clonidine induced depression of twitch response of field stimulated prostatic portion ... | J Med Chem 31: 1861-6 (1988) BindingDB Entry DOI: 10.7270/Q2HD7XVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM50020168 (1N-(2-methoxybenzyl)-6N-(2-{2-[6-(2-methoxybenzyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of Butyrylcholinesterase (BChE) of human erythrocytes [-log IC50 (uM)] | J Med Chem 41: 4186-9 (1998) Checked by Author Article DOI: 10.1021/jm9810452 BindingDB Entry DOI: 10.7270/Q2HH6KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||