

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50020375 CHEMBL3289785
SMILES: CC(C)C[C@@H]1NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CSC(=O)[C@H](Cc2ccccc2)NC1=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)CN)C(C)C
InChI Key: InChIKey=GOMDIOPXFUESDK-NWHYMTRRSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accessory gene regulator protein A (Staphylococcus aureus) | BDBM50020375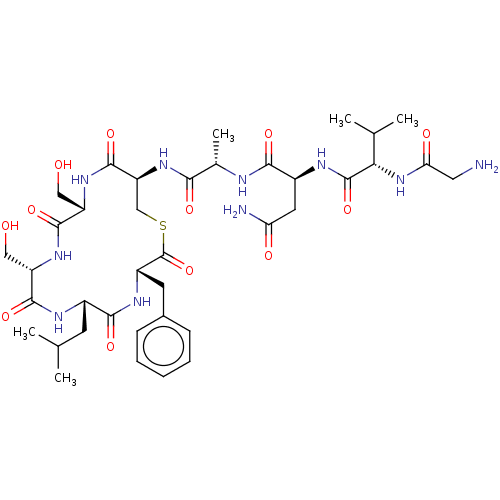 (CHEMBL3289785) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Greensboro Curated by ChEMBL | Assay Description Inhibition of AGR quorum sensing system in methicillin-resistant Staphylococcus aureus AH2759 incubated for 15 hrs by P3-LUX reporter gene assay | J Nat Prod 77: 1351-8 (2014) Article DOI: 10.1021/np5000704 BindingDB Entry DOI: 10.7270/Q21V5GJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-histidine kinase AgrC (Staphylococcus aureus) | BDBM50020375 (CHEMBL3289785) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN8463 assessed as beta-lactamase activity incubated for 80 mins by spectrophotomet... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-histidine kinase AgrC (Staphylococcus aureus) | BDBM50020375 (CHEMBL3289785) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Eastern Colorado Health Care System Curated by ChEMBL | Assay Description Inhibition of AgrC in mid-exponential phase Staphylococcus aureus RN6390B assessed as beta-lactamase activity incubated for 80 mins by spectrophotome... | J Med Chem 63: 2705-2730 (2020) Article DOI: 10.1021/acs.jmedchem.9b00798 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||