

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50020701 1-(13,16-Dibenzyl-7-carbamoylmethyl-10-isopropyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaaza-cycloicosane-4-carbonyl)-pyrrolidine-2-carboxylic acid [1-(carbamoylmethyl-carbamoyl)-4-guanidino-butyl]-amide::CHEMBL2371440
SMILES: CC(C)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(N)=O
InChI Key: InChIKey=YDDZJUBSHQSGST-CDKUOFBKSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020701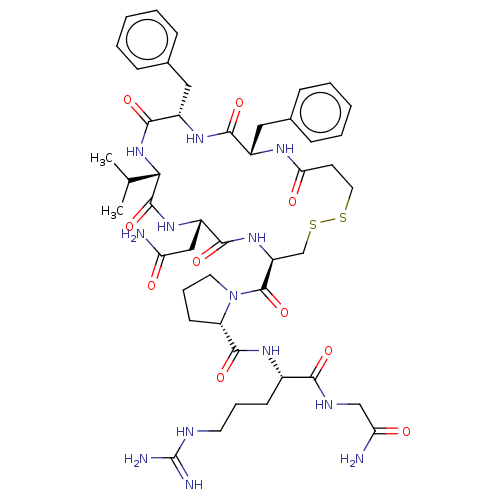 (1-(13,16-Dibenzyl-7-carbamoylmethyl-10-isopropyl-6...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparation | J Med Chem 31: 742-4 (1988) BindingDB Entry DOI: 10.7270/Q2VD6XFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||