

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1nc(cs1)-c1nc(cs1)C(=O)NCCCCC(=O)NO
InChI Key: InChIKey=YHULCWIEZUPPSB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50020885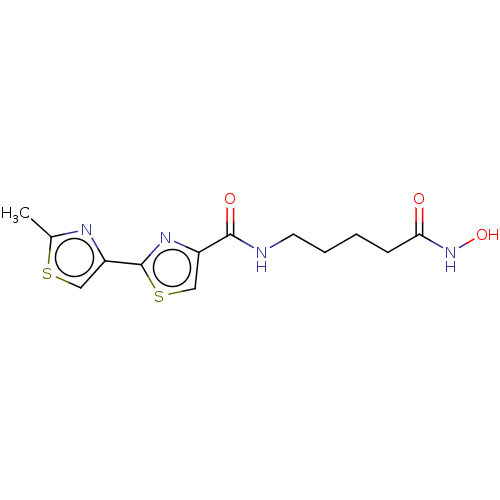 (CHEMBL3287022 | US9216962, CFH340-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Puyi Chemical Co., Ltd. US Patent | Assay Description Enzyme activity of HDAC1,3 is determined using the substrate Ac-Lys-Tyr-Lys(Ac)-AMC while the enzyme activity of HDAC6 is assayed using the substrate... | US Patent US9216962 (2015) BindingDB Entry DOI: 10.7270/Q2QJ7G3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50020885 (CHEMBL3287022 | US9216962, CFH340-4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 615 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Puyi Chemical Co., Ltd. US Patent | Assay Description Enzyme activity of HDAC1,3 is determined using the substrate Ac-Lys-Tyr-Lys(Ac)-AMC while the enzyme activity of HDAC6 is assayed using the substrate... | US Patent US9216962 (2015) BindingDB Entry DOI: 10.7270/Q2QJ7G3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50020885 (CHEMBL3287022 | US9216962, CFH340-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese National Center for Drug Screening Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC6 using Boc-Lys(epsilon-acetyl)-AMC as substrate after 3 hrs by fluorescence assay | ACS Med Chem Lett 5: 628-33 (2014) Article DOI: 10.1021/ml400470s BindingDB Entry DOI: 10.7270/Q2V40WRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50020885 (CHEMBL3287022 | US9216962, CFH340-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese National Center for Drug Screening Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC1 using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC as substrate after 24 hrs by fluorescence assay | ACS Med Chem Lett 5: 628-33 (2014) Article DOI: 10.1021/ml400470s BindingDB Entry DOI: 10.7270/Q2V40WRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50020885 (CHEMBL3287022 | US9216962, CFH340-4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese National Center for Drug Screening Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC3 using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC as substrate after 24 hrs by fluorescence assay | ACS Med Chem Lett 5: 628-33 (2014) Article DOI: 10.1021/ml400470s BindingDB Entry DOI: 10.7270/Q2V40WRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50020885 (CHEMBL3287022 | US9216962, CFH340-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Puyi Chemical Co., Ltd. US Patent | Assay Description Enzyme activity of HDAC1,3 is determined using the substrate Ac-Lys-Tyr-Lys(Ac)-AMC while the enzyme activity of HDAC6 is assayed using the substrate... | US Patent US9216962 (2015) BindingDB Entry DOI: 10.7270/Q2QJ7G3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||