 Found 8 hits for monomerid = 50022184
Found 8 hits for monomerid = 50022184 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50022184
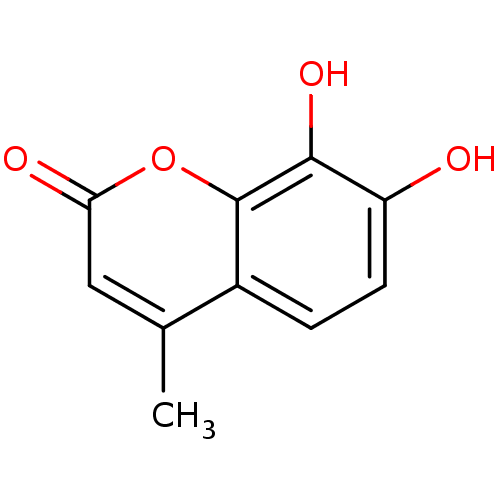
(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | -8.32 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
S.G.S.I.T.S
| Assay Description
An applied photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity. Phenol red (at a concentration of... |
J Enzyme Inhib Med Chem 29: 292-6 (2014)
Article DOI: 10.3109/14756366.2013.777334
BindingDB Entry DOI: 10.7270/Q2J38RFK |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60E+3 | -6.56 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
S.G.S.I.T.S
| Assay Description
An applied photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity. Phenol red (at a concentration of... |
J Enzyme Inhib Med Chem 29: 292-6 (2014)
Article DOI: 10.3109/14756366.2013.777334
BindingDB Entry DOI: 10.7270/Q2J38RFK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.70E+3 | -6.41 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
S.G.S.I.T.S
| Assay Description
An applied photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity. Phenol red (at a concentration of... |
J Enzyme Inhib Med Chem 29: 292-6 (2014)
Article DOI: 10.3109/14756366.2013.777334
BindingDB Entry DOI: 10.7270/Q2J38RFK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+5 | >-4.69 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
S.G.S.I.T.S
| Assay Description
An applied photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity. Phenol red (at a concentration of... |
J Enzyme Inhib Med Chem 29: 292-6 (2014)
Article DOI: 10.3109/14756366.2013.777334
BindingDB Entry DOI: 10.7270/Q2J38RFK |
More data for this
Ligand-Target Pair | |
Aldose reductase
(Rattus norvegicus) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory concentration against rat lens aldose reductase (uncompetitive inhibition type) |
J Med Chem 29: 1094-9 (1986)
BindingDB Entry DOI: 10.7270/Q20865WC |
More data for this
Ligand-Target Pair | |
Probable maltase-glucoamylase 2
(Homo sapiens) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goa University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-glucosidase (unknown origin) incubated for 20 mins before pNPG substrate addition and measured after 30 mins by UV spectrometry |
Bioorg Med Chem 27: 2340-2344 (2019)
Article DOI: 10.1016/j.bmc.2018.12.021 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50022184

(7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...)Show InChI InChI=1S/C10H8O4/c1-5-4-8(12)14-10-6(5)2-3-7(11)9(10)13/h2-4,11,13H,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data