

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50023751 19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-16-isopropyl-12,15,18,21,24-pentaoxo-7,8-dithia-11,14,17,20,23-pentaaza-spiro[5.19]pentacosane-10-carboxylic acid [(1-carbamoyl-4-guanidino-butylcarbamoyl)-methyl]-(3-guanidino-propyl)-amide::CHEMBL2372281
SMILES: [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1
InChI Key: InChIKey=RKWOSZKALALWQX-CUSBUNPBSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Sus scrofa) | BDBM50023751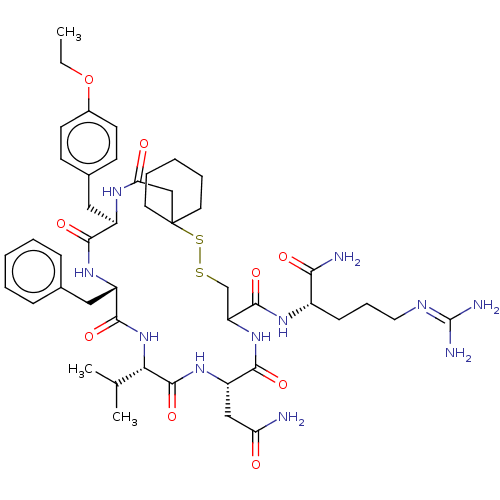 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023751 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||