

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50025946 4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H-imidazol-4-yl)-propionylamino]-5-cyclohexyl-3-hydroxy-pentanoic acid [1-(1-carbamoyl-2-phenyl-ethylcarbamoyl)-3-methyl-butyl]-amide::CHEMBL299144::CHEMBL3144410
SMILES: CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O
InChI Key: InChIKey=HMWSVKCISNXDGB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50025946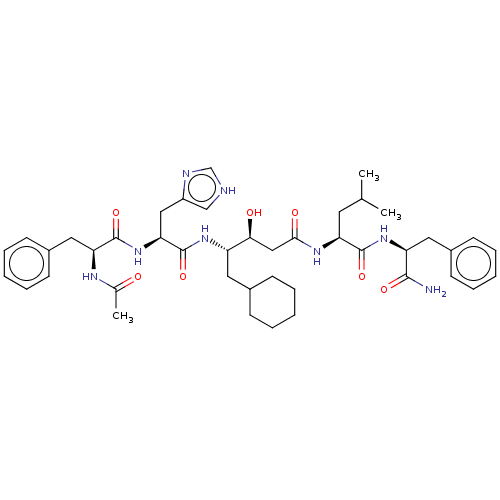 (4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human kidney renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025946 (4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha3-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025946 (4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||