

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50028866 CHEMBL297271::N-[(1S,2R)-2-(4-Hydroxy-phenyl)-indan-1-yl]-2,2-diphenyl-acetamide
SMILES: Oc1ccc(cc1)[C@H]1Cc2ccccc2[C@H]1NC(=O)C(c1ccccc1)c1ccccc1
InChI Key: InChIKey=ZPORBGGQIAIHNC-IXCJQBJRSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterol O-acyltransferase, Soat (Rattus norvegicus) | BDBM50028866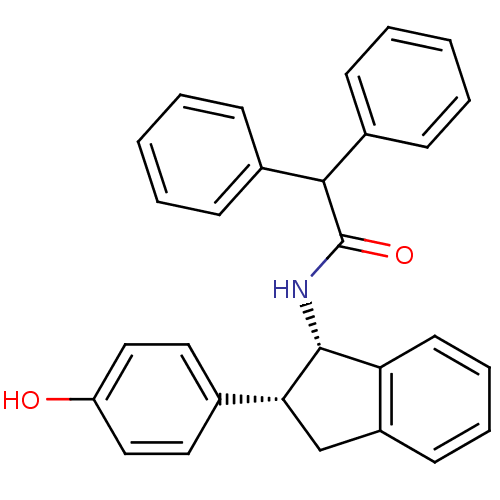 (CHEMBL297271 | N-[(1S,2R)-2-(4-Hydroxy-phenyl)-ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against Acyl coenzyme A:cholesterol acyltransferase 1 from rat hepatic microsomes at 10 microM | J Med Chem 39: 1704-19 (1996) Article DOI: 10.1021/jm950833d BindingDB Entry DOI: 10.7270/Q2QN65V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50028866 (CHEMBL297271 | N-[(1S,2R)-2-(4-Hydroxy-phenyl)-ind...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Acyl coenzyme A:cholesterol acyltransferase (ACAT) | J Med Chem 24: 496-9 (1981) BindingDB Entry DOI: 10.7270/Q2K64JTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase, Soat (Rattus norvegicus) | BDBM50028866 (CHEMBL297271 | N-[(1S,2R)-2-(4-Hydroxy-phenyl)-ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl-coenzyme A:cholesterol acyltransferase 1 from rat hepatic microsomes. | J Med Chem 39: 1704-19 (1996) Article DOI: 10.1021/jm950833d BindingDB Entry DOI: 10.7270/Q2QN65V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||