

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50029028 (cibacron blue,para isomer)1-Amino-4-{4-[4-chloro-6-(4-sulfo-phenylamino)-[1,3,5]triazin-2-ylamino]-3-sulfo-phenylamino}-9,10-dioxo-9,10-dihydro-anthracene-2-sulfonic acid
SMILES: Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4ccc(cc4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O
InChI Key: InChIKey=IYZXGLGVLYBKAI-UHFFFAOYSA-K
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choline acetylase (Homo sapiens (Human)) | BDBM50029028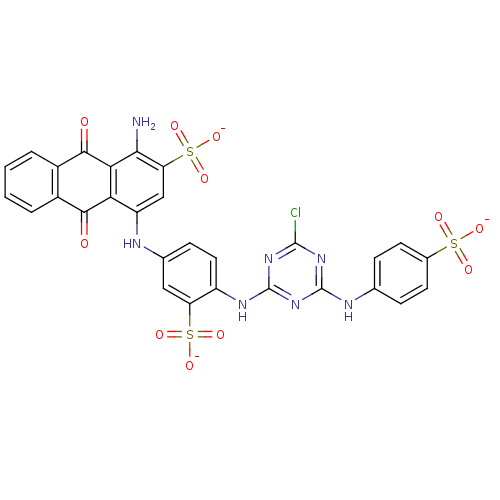 ((cibacron blue,para isomer)1-Amino-4-{4-[4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of choline acetyltransferase isolated from squid head ganglia | J Med Chem 24: 1534-7 (1982) BindingDB Entry DOI: 10.7270/Q2T43TMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apurinic-apyrimidinic endonuclease 1 (APE-1) (Homo sapiens (Human)) | BDBM50029028 ((cibacron blue,para isomer)1-Amino-4-{4-[4-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Russian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human APE1 preincubated for 15 mins followed by substrate addition by HTS assay | Bioorg Med Chem 25: 2531-2544 (2017) Article DOI: 10.1016/j.bmc.2017.01.028 BindingDB Entry DOI: 10.7270/Q2P55R4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y4 (Homo sapiens (Human)) | BDBM50029028 ((cibacron blue,para isomer)1-Amino-4-{4-[4-chloro-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2Y4 receptor transfected in human 1321N1 cells assessed as inhibition of UTP-activated intracellular calcium mobilizati... | J Med Chem 60: 3020-3038 (2017) Article DOI: 10.1021/acs.jmedchem.7b00030 BindingDB Entry DOI: 10.7270/Q2G73H15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||