

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50029352 CHEMBL336033::[(5S,8S,11R,14S,16aR)-8-(1H-Indol-3-ylmethyl)-11-isobutyl-14-isopropyl-4,7,10,13,16-pentaoxo-hexadecahydro-3a,6,9,12,15-pentaaza-cyclopentacyclopentadecen-5-yl]-acetic acid
SMILES: CC(C)C[C@H]1NC(=O)[C@@H](NC(=O)[C@H]2CCCN2C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C
InChI Key: InChIKey=VYCMAAOURFJIHD-XPICPDFBSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor ET-B (Sus scrofa) | BDBM50029352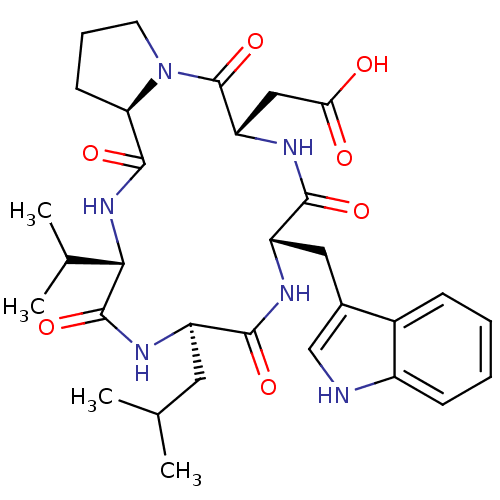 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against porcine Endothelin B receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EDNRB (Homo sapiens (Human)) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against Human Endothelin B receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor ET-A (Sus scrofa) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor ET-A (Sus scrofa) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against porcine Endothelin A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EDNRA (RAT) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University Curated by ChEMBL | Assay Description Displacement of [125I]ET-1 from rat ETA receptor after 1 hr by Lowry method | Bioorg Med Chem 23: 657-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.003 BindingDB Entry DOI: 10.7270/Q28K7BRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against Human Endothelin A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||