

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50029603 CHEMBL287919::L-745337::L745,337::N-[6-(2,4-Difluoro-phenylsulfanyl)-1-oxo-indan-5-yl]-methanesulfonamide
SMILES: CS(=O)(=O)Nc1cc2CCC(=O)c2cc1Sc1ccc(F)cc1F
InChI Key: InChIKey=HDUWKQUHMUSICC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50029603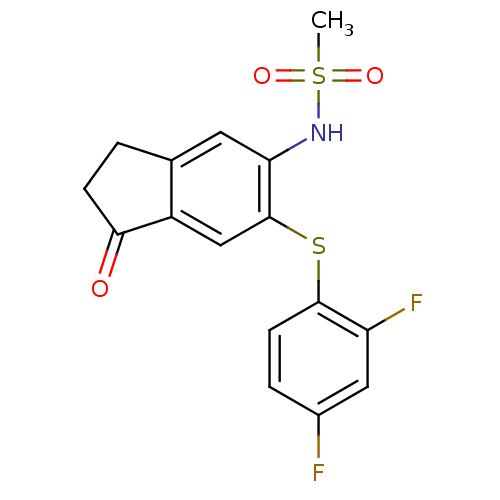 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 96: 7563-8 (1999) Article DOI: 10.1073/pnas.96.13.7563 BindingDB Entry DOI: 10.7270/Q21G0JT4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 96: 7563-8 (1999) Article DOI: 10.1073/pnas.96.13.7563 BindingDB Entry DOI: 10.7270/Q21G0JT4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 96: 7563-8 (1999) Article DOI: 10.1073/pnas.96.13.7563 BindingDB Entry DOI: 10.7270/Q21G0JT4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 1 in human U937 cells | J Med Chem 38: 4897-905 (1996) BindingDB Entry DOI: 10.7270/Q28051PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of prostaglandin G/H synthase 2 (COX-2). | J Med Chem 38: 4570-8 (1995) BindingDB Entry DOI: 10.7270/Q23N22D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of prostaglandin G/H synthase 2 expressed in human osteosarcoma 143 cells | J Med Chem 38: 4897-905 (1996) BindingDB Entry DOI: 10.7270/Q28051PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 1 activity in U937 microsomal assay | Bioorg Med Chem Lett 9: 151-6 (1999) BindingDB Entry DOI: 10.7270/Q21G0KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of purified recombinant human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 9: 151-6 (1999) BindingDB Entry DOI: 10.7270/Q21G0KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human Prostaglandin G/H synthase 2 expressed in CHO (chinese hamster ovary) cells | Bioorg Med Chem Lett 9: 151-6 (1999) BindingDB Entry DOI: 10.7270/Q21G0KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase 1 activity in U937 microsomal assay | Bioorg Med Chem Lett 9: 151-6 (1999) BindingDB Entry DOI: 10.7270/Q21G0KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50029603 (CHEMBL287919 | L-745337 | L745,337 | N-[6-(2,4-Dif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of prostaglandin G/H synthase 1. | J Med Chem 38: 4570-8 (1995) BindingDB Entry DOI: 10.7270/Q23N22D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||