

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50030732 CHEMBL323506::N-{3-[3-Butyl-4-(3-fluoro-2'(tert-butoxycarbonyl) sulfamoyl-5'-propyl-biphenyl-4-ylmethyl)-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-4-chloro-phenyl}-acetamide::N-{3-[3-Butyl-4-(3-fluoro-5'-propyl-2'-(N-t-butyloxycarbonyl)-Sulfanamido-biphenyl-4-ylmethyl)-5-oxo-4,5-dihydro-[1,2,4]triazol-1-yl]-4-chloro-phenyl}-acetamide
SMILES: CCCCc1nn(-c2cc(NC(C)=O)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1cc(CCC)ccc1S(=O)(=O)NC(=O)OC(C)(C)C
InChI Key: InChIKey=OVGREWSEMONLCV-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II AT2 (RAT) | BDBM50030732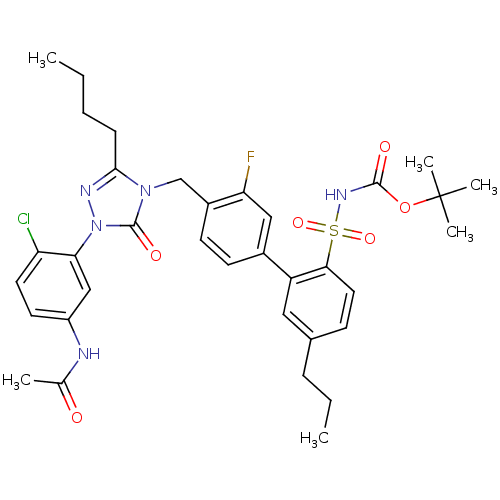 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT2 receptor from rat midbrain | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against cloned human AT1 receptor | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II AT2 (RAT) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 2 in rat midbrain membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (AT-1) type-1 (RAT) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against AT1 receptor from rat adrenal tissues. | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against AT1 receptor from human adrenal tissues. | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against AT2 receptor from human adrenal tissues. | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (RAT) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against AT1 receptor from rat adrenal tissues. | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50030732 (CHEMBL323506 | N-{3-[3-Butyl-4-(3-fluoro-2'(tert-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT1 receptor from rabbit aorta | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||