

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50031709 4-Guanidino-benzoic acid 4-carboxymethyl-phenyl ester; compound with methanesulfonic acid::CHEMBL433135
SMILES: [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc(-[#6]-[#6](-[#8])=O)cc1
InChI Key: InChIKey=XTKGXPFBKPYFDW-UHFFFAOYSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kallikrein-1 (KLK1) (Homo sapiens (Human)) | BDBM50031709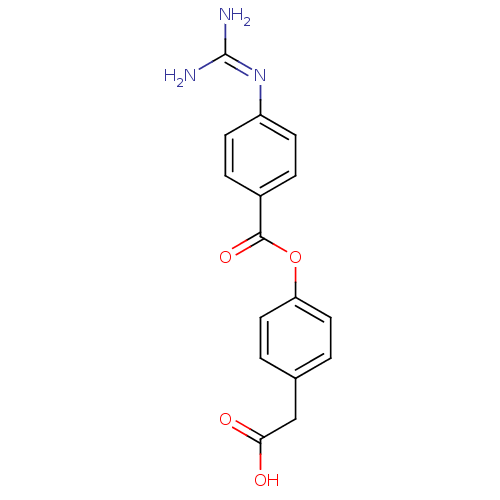 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Val-Leu-Arg-pNA) for pancreatic kallikrein in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (KLK1) (Homo sapiens (Human)) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Pro-Phe-Arg-pNA) for plasma kallikrein in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Phe-Pip-Arg-pNA) thrombin in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (MeO-Suc-Ala-Ala-Pro-Val-pNA) for sputum elastase in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-chymotrypsin (Bos taurus (bovine)) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (Suc-Ala-Ala-Pro-Phe-pNA) for chymotrypsin in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmin (Rattus norvegicus) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (H-D-Val-Leu-Lys-pNA) for plasmin in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (Boc-Phe-Ser-Arg-AMC) for trypsin in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50031709 (4-Guanidino-benzoic acid 4-carboxymethyl-phenyl es...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit enzymatic cleavage of the chromogenic substrate (MeO-Suc-Ala-Ala-Pro-Met-pNA) for cathepsin G in vitro. | J Med Chem 38: 2521-3 (1995) BindingDB Entry DOI: 10.7270/Q2MC8Z12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||