

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50032193 (S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propionylamino)-5-amino-pentanoylamino]-N-((1S,2S)-1-{(1S,2S)-1-[(S)-1-carboxy-2-(1H-indol-3-yl)-ethylcarbamoyl]-2-methyl-butylcarbamoyl}-2-methyl-butyl)-succinamic acid::CHEMBL94983
SMILES: CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCN)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI Key: InChIKey=IEJDKICPBODJMQ-TYTHOBKFSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032193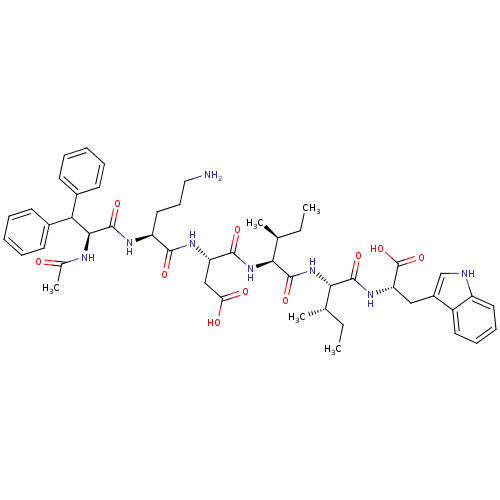 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor from rabbit renal vascular smooth muscles | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EDNRB (RAT) | BDBM50032193 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against endothelin B receptor rat cerebellar membranes. | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50032193 ((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of ET-1 stimulated arachidonic acid release in rabbit renal vascular smooth muscle cells | J Med Chem 38: 2809-19 (1995) BindingDB Entry DOI: 10.7270/Q2KD1WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||