

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50033061 27-Hydroxymethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-30-propyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone::CHEMBL384726
SMILES: CCC[C@H]1NC(=O)[C@@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@@H](C(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](C)NC(=O)[C@@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CO)N(C)C1=O)C(C)C
InChI Key: InChIKey=UVAZRAAUMKLNAN-INJCWPFZSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50033061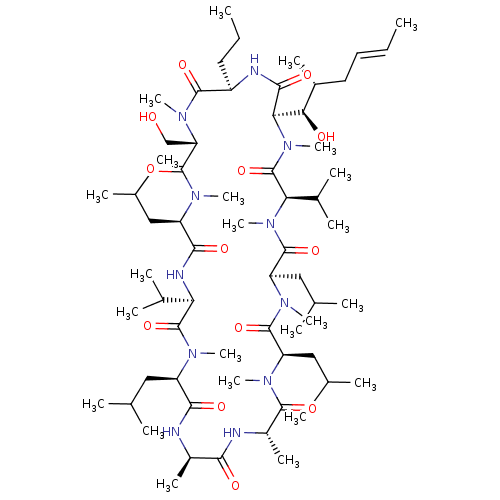 (27-Hydroxymethyl-33-(1-hydroxy-2-methyl-hex-4-enyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against cyclophilin A by rotamase assay | J Med Chem 38: 3361-7 (1995) BindingDB Entry DOI: 10.7270/Q27W6B79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-2 (Mus musculus) | BDBM50033061 (27-Hydroxymethyl-33-(1-hydroxy-2-methyl-hex-4-enyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description The compound was evaluated in vitro for the immunosuppressive activity in interleukin-2 by interleukin-2 reporter gene assay (IL2-RGA) | J Med Chem 38: 3361-7 (1995) BindingDB Entry DOI: 10.7270/Q27W6B79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50033061 (27-Hydroxymethyl-33-(1-hydroxy-2-methyl-hex-4-enyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against cyclophilin A by ELISA | J Med Chem 38: 3361-7 (1995) BindingDB Entry DOI: 10.7270/Q27W6B79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||