

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50034220 2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethylethanaminium hydroxide::CHEMBL125::MILTEFOSINE::[2-(Hexadecyloxy-hydroxy-phosphoryloxy)-ethyl]-trimethyl-ammonium::hexadecyl 2-(trimethyl-lambda~5~-azanyl)ethyl hydrogen phosphate::hexadecyl 2-(trimethylammonio)ethyl phosphate::hexadecyloxy-2-trimethylammonioethylphosphorate::hexadecylphosphocholine::hexadecylphosphocholine, miltefosine::n-hexadecylphosphocholine
SMILES: CCCCCCCCCCCCCCCCOP([O-])(=O)OCC[N+](C)(C)C
InChI Key: InChIKey=PQLXHQMOHUQAKB-UHFFFAOYSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphoehtnaolamine Methyltransferases 1 (PMT1) (Caenorhabditis elegans) | BDBM50034220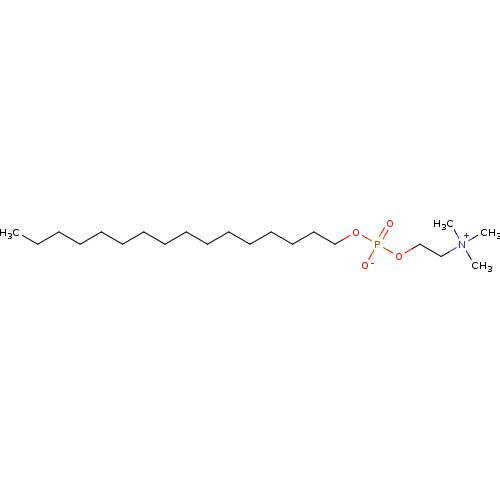 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Washington University | Assay Description A radiochemical assay was used to measure enzymatic activity. | J Biol Chem 286: 38060-8 (2011) Article DOI: 10.1074/jbc.M111.290619 BindingDB Entry DOI: 10.7270/Q2GF0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoehtnaolamine Methyltransferases 2 (PMT2) (Caenorhabditis elegans) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Washington University | Assay Description A radiochemical assay was used to measure enzymatic activity. | J Biol Chem 286: 38060-8 (2011) Article DOI: 10.1074/jbc.M111.290619 BindingDB Entry DOI: 10.7270/Q2GF0S39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet activating factor receptor (Cavia porcellus) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | 37 |
Laboratorios Menarini SA Curated by ChEMBL | Assay Description PAF agonism was measured as the IC50 for aggregation of washed rabbit platelets after incubation for 30 min at 37 C. | J Med Chem 38: 1216-28 (1995) BindingDB Entry DOI: 10.7270/Q2862H3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of Cell division cycle 2 (cdc2) kinase | J Med Chem 39: 2609-14 (1996) Article DOI: 10.1021/jm9509152 BindingDB Entry DOI: 10.7270/Q2G15ZXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| phosphatase Cdc25 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of Cell division cycle 25 (Cdc25) phosphatase | J Med Chem 39: 2609-14 (1996) Article DOI: 10.1021/jm9509152 BindingDB Entry DOI: 10.7270/Q2G15ZXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of Akt phosphorylation in human insulin-stimulated A549 cells incubated for 2 hrs prior to insulin-induction measured after 30 mins by ELI... | Bioorg Med Chem 21: 2018-24 (2013) Article DOI: 10.1016/j.bmc.2013.01.010 BindingDB Entry DOI: 10.7270/Q2NG4S1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP2D6 expressed in baculovirus infected BTI-TN-5B1-4 cells incubated for 30 mins by luciferase assay | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP3A4 expressed in baculovirus infected BTI-TN-5B1-4 cells incubated for 30 mins by luciferase assay | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of recombinant full-length human aurora B kinase expressed in baculovirus system using MBP as substrate incubated for 45 mins by ADP-glo k... | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP2C9 expressed in baculovirus infected BTI-TN-5B1-4 cells incubated for 30 mins by luciferase assay | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP2C19 expressed in baculovirus infected BTI-TN-5B1-4 cells incubated for 30 mins by luciferase assay | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2 expressed in baculovirus infected BTI-TN-5B1-4 cells incubated for 30 mins by luciferase assay | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of human ERG incubated for 2 hrs by fluorescence polarisation assay | Eur J Med Chem 146: 423-434 (2018) Article DOI: 10.1016/j.ejmech.2018.01.043 BindingDB Entry DOI: 10.7270/Q2SN0CMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Hex B) (Homo sapiens (Human)) | BDBM50034220 (2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GRI BIO, INC. US Patent | Assay Description Buffers and solutions: Phosphate buffered saline (PBS) and 1 M HEPES were provided by the in-house service facility. Tyrode's buffer (TyB) consis... | US Patent US10143668 (2018) BindingDB Entry DOI: 10.7270/Q2K35WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||