

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50036051 ((S)-1-{(S)-2-[(S)-1-(Benzothiazole-2-carbonyl)-2-methyl-propylcarbamoyl]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-carbamic acid benzyl ester::CHEMBL353441::benzyl (S)-1-((S)-2-(((S)-1-(benzo[d]thiazol-2-yl)-3-methyl-1-oxobutan-2-yl)carbamoyl)pyrrolidin-1-yl)-3-methyl-1-oxobutan-2-ylcarbamate
SMILES: CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)c1nc2ccccc2s1
InChI Key: InChIKey=CRBPOVQLGBBSBX-HVCNVCAESA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50036051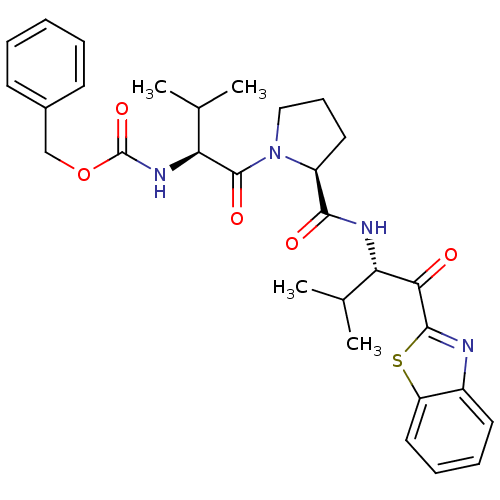 (((S)-1-{(S)-2-[(S)-1-(Benzothiazole-2-carbonyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50036051 (((S)-1-{(S)-2-[(S)-1-(Benzothiazole-2-carbonyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | J Med Chem 54: 2529-91 (2011) Article DOI: 10.1021/jm1013693 BindingDB Entry DOI: 10.7270/Q24M95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50036051 (((S)-1-{(S)-2-[(S)-1-(Benzothiazole-2-carbonyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||