

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50036133 7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1,3]dioxolo[4,5-g]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-8,11-dione::7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1,3]dioxolo[5,4-g]pyrano[3'',4'':6,7]indolizino[1,2-b]quinoline-8,11-dione::CHEMBL307794
SMILES: CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4cc5OCOc5cc4cc3Cn1c2=O
InChI Key: InChIKey=RPFYDENHBPRCTN-NRFANRHFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM50036133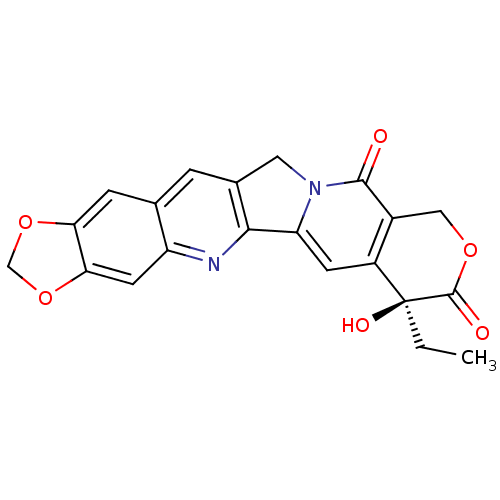 (7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute Curated by ChEMBL | Assay Description Inhibition of topoisomerase I activity was determined in vitro by using the cleavable complex assay(calf thymus) | J Med Chem 38: 395-401 (1995) BindingDB Entry DOI: 10.7270/Q2F47N67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM50036133 (7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of Topoisomerase I by cleavage complex formation in human HL-60 cells | J Med Chem 36: 2689-700 (1993) BindingDB Entry DOI: 10.7270/Q2KP82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM50036133 (7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit calf thymus topoisomerase I when assayed in the cleavable complex formation | Bioorg Med Chem Lett 5: 2129-2132 (1995) Article DOI: 10.1016/0960-894X(95)00360-6 BindingDB Entry DOI: 10.7270/Q20Z7379 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM50036133 (7-ethyl-7-hydroxy-(7S)-7,8,11,13-tetrahydro-10H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Effective concentration against DNA topoisomerase I | Bioorg Med Chem Lett 14: 5377-81 (2004) Article DOI: 10.1016/j.bmcl.2004.08.010 BindingDB Entry DOI: 10.7270/Q2D799WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||