

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50037833 (3S,4aS,8aS)-2-[(S)-3-({(2R,4S)-2-[(4-Dimethylamino-benzylcarbamoyl)-((S)-phenylacetylamino)-methyl]-5,5-dimethyl-thiazolidine-4-carbonyl}-amino)-2-hydroxy-propyl]-decahydro-isoquinoline-3-carboxylic acid tert-butylamide::CHEMBL333982
SMILES: CN(C)c1ccc(CNC(=O)C(NC(=O)Cc2ccccc2)[C@@H]2N[C@@H](C(=O)NC[C@H](O)CN3C[C@H]4CCCC[C@H]4C[C@H]3C(=O)NC(C)(C)C)C(C)(C)S2)cc1
InChI Key: InChIKey=WIXUDGGEVYMHOW-VYWPSWJASA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50037833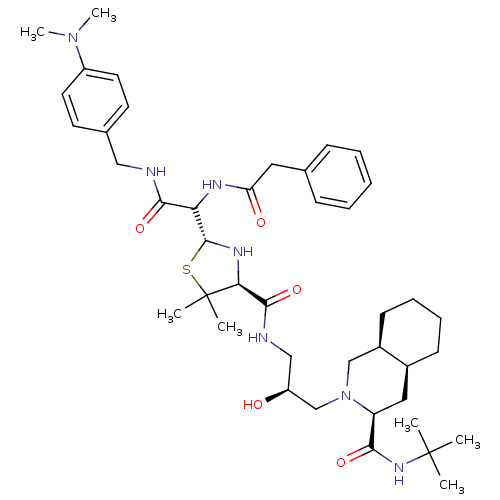 ((3S,4aS,8aS)-2-[(S)-3-({(2R,4S)-2-[(4-Dimethylamin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Limited Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV proteinase | J Med Chem 37: 3707-16 (1994) BindingDB Entry DOI: 10.7270/Q2V40T88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||