 Found 8 hits for monomerid = 50038066
Found 8 hits for monomerid = 50038066 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50038066
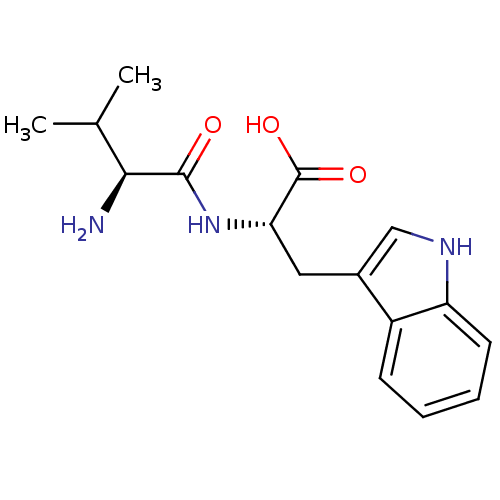
((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteus Molecular Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition against ACE. |
J Med Chem 37: 3994-4002 (1994)
BindingDB Entry DOI: 10.7270/Q2XW4HWB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tianjin University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) |
Eur J Med Chem 84: 100-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.015
BindingDB Entry DOI: 10.7270/Q29025F2 |
More data for this
Ligand-Target Pair | |
Oligopeptide transporter small intestine isoform
(Homo sapiens (Human)) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human PEPT1 assessed as inhibition of [14C]Gly-Sar uptake in MDCK cells |
J Med Chem 49: 3636-44 (2006)
Article DOI: 10.1021/jm0511029
BindingDB Entry DOI: 10.7270/Q2P55P9R |
More data for this
Ligand-Target Pair | |
Thermolysin
(Bacillus thermoproteolyticus) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by ChEMBL
| Assay Description
Binding affinity against Thermolysin |
J Med Chem 45: 2770-80 (2002)
Article DOI: 10.1021/jm0105833
BindingDB Entry DOI: 10.7270/Q2MG7S8S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Biological activity was measured against Angiotensin I converting enzyme |
J Med Chem 38: 2705-13 (1995)
BindingDB Entry DOI: 10.7270/Q2GX4CSN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity on Angiotensin I converting enzyme (ACE) obtained from pig renal cortex and hippuryl-histidyl-leucine as substrate |
J Med Chem 32: 289-97 (1989)
BindingDB Entry DOI: 10.7270/Q2XP75JS |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) using Gly-Pro-pNA as substrate after 60 mins |
Eur J Med Chem 151: 145-157 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.041
BindingDB Entry DOI: 10.7270/Q2CZ39SV |
More data for this
Ligand-Target Pair | |
Oligopeptide transporter small intestine isoform
(Homo sapiens (Human)) | BDBM50038066

((S)-2-((S)-2-Amino-3-methyl-butyrylamino)-3-(1H-in...)Show SMILES CC(C)[C@H](N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C16H21N3O3/c1-9(2)14(17)15(20)19-13(16(21)22)7-10-8-18-12-6-4-3-5-11(10)12/h3-6,8-9,13-14,18H,7,17H2,1-2H3,(H,19,20)(H,21,22)/t13-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Activation of human PEPT1 expressed in MDCK cells |
J Med Chem 49: 3636-44 (2006)
Article DOI: 10.1021/jm0511029
BindingDB Entry DOI: 10.7270/Q2P55P9R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data