 Found 12 hits for monomerid = 50038843
Found 12 hits for monomerid = 50038843 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C21
(Mus musculus) | BDBM50038843
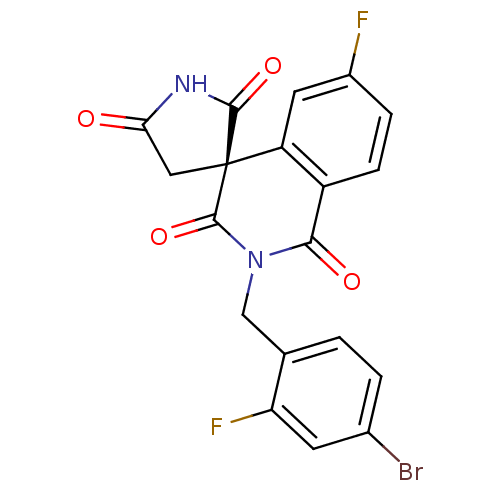
((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AKR1C21 |
Bioorg Med Chem 16: 3245-54 (2008)
Article DOI: 10.1016/j.bmc.2007.12.016
BindingDB Entry DOI: 10.7270/Q2PZ59P2 |
More data for this
Ligand-Target Pair | |
Aldose reductase (AR)
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 72.6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Eye Institute
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Human recombinant Aldose Reductase (wild type) |
J Med Chem 43: 1062-70 (2000)
BindingDB Entry DOI: 10.7270/Q2QZ2BPN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldose reductase (AR)
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia
Curated by ChEMBL
| Assay Description
Inhibition of aldose reductase |
Bioorg Med Chem 15: 7865-77 (2007)
Article DOI: 10.1016/j.bmc.2007.08.019
BindingDB Entry DOI: 10.7270/Q2QR4WV6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C21
(Mus musculus) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AKR1C21 |
Bioorg Med Chem 16: 3245-54 (2008)
Article DOI: 10.1016/j.bmc.2007.12.016
BindingDB Entry DOI: 10.7270/Q2PZ59P2 |
More data for this
Ligand-Target Pair | |
Aldose reductase (AR)
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant aldose reductase |
Bioorg Med Chem 16: 3245-54 (2008)
Article DOI: 10.1016/j.bmc.2007.12.016
BindingDB Entry DOI: 10.7270/Q2PZ59P2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldose reductase (AR)
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Compound was evaluated In vitro for inhibition of aldose reductase activity by 50% in dog RBC |
J Med Chem 37: 2043-58 (1994)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2BR8STM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldose reductase (ALR2)
(Bos taurus (Cattle)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of aldose reductase activity in a partially purified bovine lens preparation |
J Med Chem 37: 2043-58 (1994)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2BR8STM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family member 1B10 (AKR1B10)
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of reductase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of NADPH linked pyr... |
Bioorg Med Chem 18: 2485-90 (2010)
Article DOI: 10.1016/j.bmc.2010.02.050
BindingDB Entry DOI: 10.7270/Q2K35TTH |
More data for this
Ligand-Target Pair | |
Aldose reductase (AR)
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged human aldose reductase expressed in Escherichia coli BL21(DE3) mediated NADPH linked pyridine-3-aldehyde reducti... |
Bioorg Med Chem 18: 2485-90 (2010)
Article DOI: 10.1016/j.bmc.2010.02.050
BindingDB Entry DOI: 10.7270/Q2K35TTH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde reductase
(Homo sapiens (Human)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6His-tagged human aldehyde reductase expressed in Escherichia coli BL21(DE3) mediated D-glucuronate reduction |
Bioorg Med Chem 18: 2485-90 (2010)
Article DOI: 10.1016/j.bmc.2010.02.050
BindingDB Entry DOI: 10.7270/Q2K35TTH |
More data for this
Ligand-Target Pair | |
Aldose reductase (ALR2)
(Bos taurus (Cattle)) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of aldose reductase activity in a partially purified bovine lens preparation |
J Med Chem 37: 2043-58 (1994)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2BR8STM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde reductase
(Sus scrofa) | BDBM50038843

((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...)Show SMILES Fc1ccc2C(=O)N(Cc3ccc(Br)cc3F)C(=O)[C@]3(CC(=O)NC3=O)c2c1 |r| Show InChI InChI=1S/C19H11BrF2N2O4/c20-10-2-1-9(14(22)5-10)8-24-16(26)12-4-3-11(21)6-13(12)19(18(24)28)7-15(25)23-17(19)27/h1-6H,7-8H2,(H,23,25,27)/t19-/m1/s1 | PDB
MMDB
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of pig ALR1 |
Bioorg Med Chem 17: 1244-50 (2009)
Article DOI: 10.1016/j.bmc.2008.12.024
BindingDB Entry DOI: 10.7270/Q20Z734Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data