

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [NH3+]C(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC([O-])=O)C([O-])=O
InChI Key: InChIKey=IQPLOQWSCZRUCA-UHFFFAOYSA-M
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039111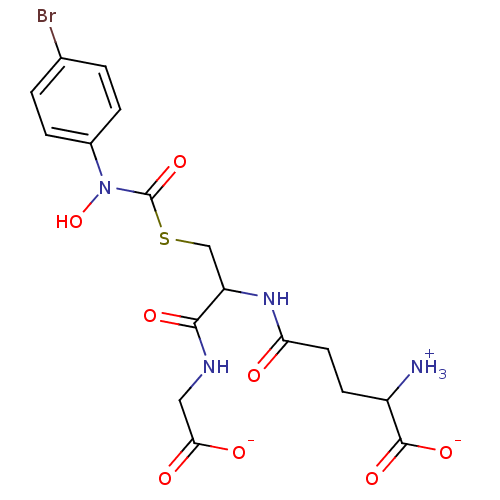 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against human erythrocyte glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039111 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50039111 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Tested for inhibitory activity against yeast glyoxalase I | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||