

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50039288 (1S,9aR,11aS)-5,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4,5,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[i]phenanthridine-1-carboxylic acid benzhydryl-amide::CHEMBL306554
SMILES: CN1CC2C3CC[C@H](C(=O)NC(c4ccccc4)c4ccccc4)[C@@]3(C)CCC2[C@@]2(C)CCC(=O)C=C12
InChI Key: InChIKey=ZZTHGPFXLVWJQY-TZSNOKOGSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50039288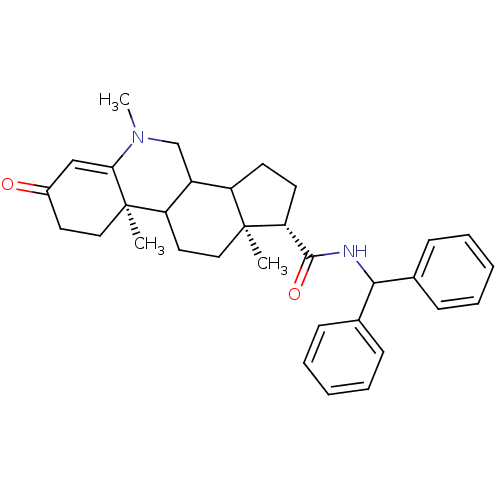 ((1S,9aR,11aS)-5,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 5-alpha-reductase (Homo sapiens (Human)) | BDBM50039288 ((1S,9aR,11aS)-5,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 2 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||