

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50040795 CHEMBL3353193::US10023562, Example 123::US10544133, Example 123::US9657009, 123
SMILES: CC(C)Nc1cc(Nc2ncc(Cl)cc2F)ncc1C(=O)NC[C@@H](F)C(C)(C)O
InChI Key: InChIKey=JOKNBOUVTOTGOG-OAHLLOKOSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50040795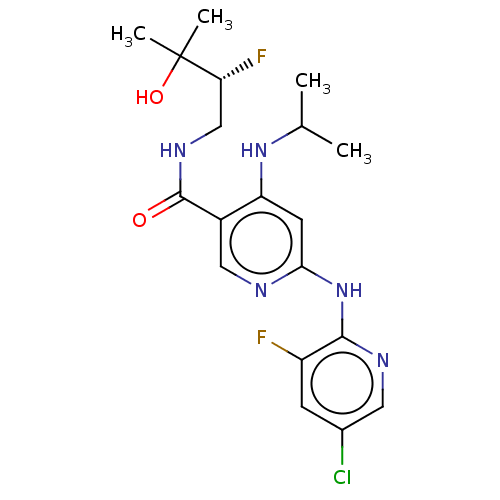 (CHEMBL3353193 | US10023562, Example 123 | US105441...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Discovery Curated by ChEMBL | Assay Description Inhibition of IRAK4 (unknown origin) using fluoresceinated peptide and ATP after 60 mins by by Caliper assay | J Med Chem 58: 96-110 (2015) Article DOI: 10.1021/jm5016044 BindingDB Entry DOI: 10.7270/Q2P84DHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50040795 (CHEMBL3353193 | US10023562, Example 123 | US105441...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10544133 (2020) BindingDB Entry DOI: 10.7270/Q23R0W8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50040795 (CHEMBL3353193 | US10023562, Example 123 | US105441...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US9657009 (2017) BindingDB Entry DOI: 10.7270/Q2D79DGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50040795 (CHEMBL3353193 | US10023562, Example 123 | US105441...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.2 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10023562 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||