

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCCCc1nn(c(C(=O)OCC)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1cc(Cl)ccc1Cl
InChI Key: InChIKey=IHEMKWIKEIESJN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidase 1 (GUINEA PIG) | BDBM50042574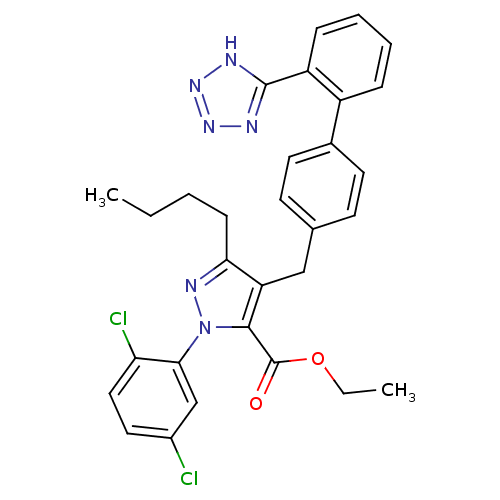 (5-Butyl-2-(2,5-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042574 (5-Butyl-2-(2,5-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacteroides fragilis) | BDBM50042574 (5-Butyl-2-(2,5-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of metallo-beta-lactamase (MBL) from Bacteroides fragilis. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM50042574 (5-Butyl-2-(2,5-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of metallo-beta-lactamase (MBL) from Pseudomonas aeruginosa mediated by the plasmid-borne IMP-1 enzyme. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||