

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50043724 (S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7-benzyl-14,14-dimethyl-6,9,12-trioxo-1,2-dithia-5,8,11-triaza-cyclotetradecane-4-carbonyl}-amino)-3-phenyl-propionic acid::CHEMBL339320
SMILES: CC1(C)SSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI Key: InChIKey=SNEMTHOGAFLXTE-OLAIZJASSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50043724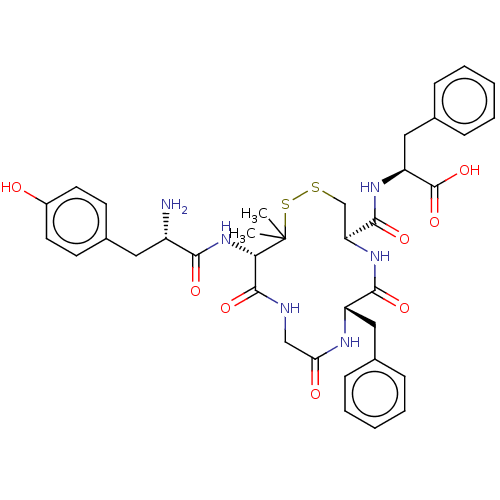 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand | J Med Chem 37: 146-50 (1994) BindingDB Entry DOI: 10.7270/Q2TX3G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor was determined in rat brain using [3H]CTOP as radioligand | J Med Chem 37: 146-50 (1994) BindingDB Entry DOI: 10.7270/Q2TX3G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration against electrically evoked contractions of guinea pig ileum longitudinal muscle-myenteric plexus | J Med Chem 37: 146-50 (1994) BindingDB Entry DOI: 10.7270/Q2TX3G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H]CTOP to Opioid receptor mu 1 in rat brain | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory potency against Opioid receptor mu 1 was determined in electrically induced strips of guinea pig ileum (GPI) | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory potency against Opioid receptor delta 1 was determined in electrically induced smooth muscle contractions of mouse vas deferens (MVD) | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50043724 ((S)-2-({(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibitory concentration against delta opioid receptor of electrically induced smooth muscle contraction of mouse vas deferens | J Med Chem 37: 146-50 (1994) BindingDB Entry DOI: 10.7270/Q2TX3G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||