

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50045885 CHEMBL100256::N,N-Dipropyl-2-(2-thiophen-2-yl-1H-indol-3-yl)-acetamide
SMILES: CCCN(CCC)C(=O)Cc1c([nH]c2ccccc12)-c1cccs1
InChI Key: InChIKey=KVWYMXMHIPUYMW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral-Type Benzodiazepine Receptor (Rattus norvegicus (rat)) | BDBM50045885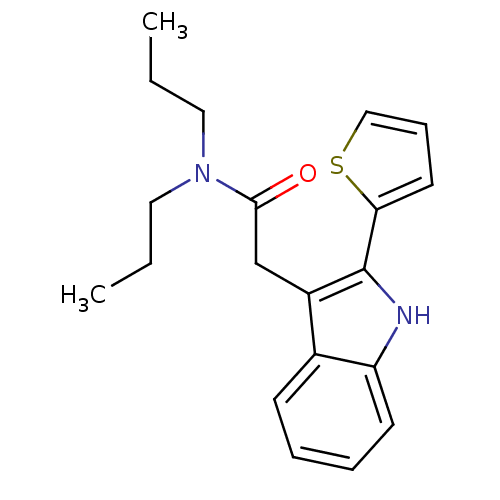 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Binding affinity against mitochondrial DBI complex (peripheral benzodiazepine receptor) using primary cultures of glial cells | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CCKBR (RAT) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity by measuring its ability to displace [3H]L-365,260 from cholecystokinin receptor in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Rattus norvegicus (rat)-RAT) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibition of [3H]naxolone binding to opiate receptors in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 1 (5-HT1) receptor (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity by measuring its ability to displace [3H]ketanserin from serotonin receptor in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor beta (Rattus norvegicus (Rat)) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity by measuring its ability to displace [125I]pindolol from beta-adrenergic receptor in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor (Rattus norvegicus (rat)-RAT) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibitory activity by measuring its ability to displace [3H]3-PPP from opiate receptor in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha (Rattus norvegicus (rat)-Rattus norvegicus (Rat)-RA...) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity by measuring its ability to displace [3H]clonidine from alpha-adrenergic receptor in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity, measured by displacement [3H]-CP-55,940 from cannabinoid receptor (CB1) in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha (Rattus norvegicus (rat)-Rattus norvegicus (Rat)-RA...) | BDBM50045885 (CHEMBL100256 | N,N-Dipropyl-2-(2-thiophen-2-yl-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Inhibitory activity by measuring its ability to displace [3H]WB-4101 from alpha-adrenergic receptor in rat brain | J Med Chem 36: 2908-20 (1993) BindingDB Entry DOI: 10.7270/Q2C24VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||