

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50048066 CHEMBL1316265::OMDM2::US10300048, Compound OMDM-2
SMILES: CCCCCCCC\C=C/CCCCCCCC(=O)N[C@@H](CO)Cc1ccc(O)cc1
InChI Key: InChIKey=ICDMLAQPOAVWNH-HAAQQRBASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver fatty acid binding protein (FABP1) (Mus musculus (Mouse)) | BDBM50048066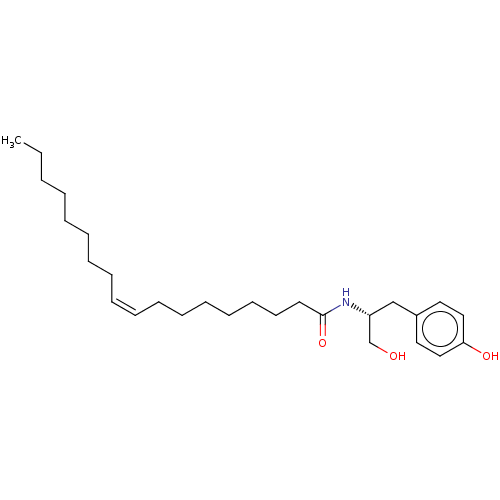 (CHEMBL1316265 | OMDM2 | US10300048, Compound OMDM-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avanti Polar Lipids | Assay Description The following fluorescent ligand displacement assays at 24 °C were used to further confirm and/or determine if the cytosolic lipidic ligand "chaperon... | Biochemistry 55: 5243-55 (2016) Article DOI: 10.1021/acs.biochem.6b00446 BindingDB Entry DOI: 10.7270/Q2MS3RJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50048066 (CHEMBL1316265 | OMDM2 | US10300048, Compound OMDM-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. | Assay Description Screening for AEA cellular uptake inhibition was performed in a semi-automated procedure: Pipetting and washing steps were performed by a Biomek3000 ... | J Med Chem 52: 1723-30 (2009) BindingDB Entry DOI: 10.7270/Q22J6F63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50048066 (CHEMBL1316265 | OMDM2 | US10300048, Compound OMDM-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. | Assay Description Hydrolysis of [3H]-AEA by FAAH was determined as previously described in cell homogenates of U937 cells (0.18 mg protein) (Omeir et al., 1999, Bioche... | J Med Chem 52: 1723-30 (2009) BindingDB Entry DOI: 10.7270/Q22J6F63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50048066 (CHEMBL1316265 | OMDM2 | US10300048, Compound OMDM-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Szeged Curated by ChEMBL | Assay Description Inhibition of FAAH-mediated [ethanol-amine-1-3H]AEA hydrolysis in human U937 cells preincubated for 15 mins measured 15 mins post AEA addition by liq... | J Nat Prod 77: 1663-9 (2014) Article DOI: 10.1021/np500292g BindingDB Entry DOI: 10.7270/Q269757F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||