

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049243 5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazolidine-2,4-dione::5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazolidine-2,4-dione (ciglitazone)::5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazolidine-2,4-dione(Ciglitazone)::CHEMBL7002::CIGLITAZONE::US8637558, 112
SMILES: CC1(COc2ccc(Cc3sc(=O)[nH]c3O)cc2)CCCCC1
InChI Key: InChIKey=YNQLJZYIOAPXMU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50049243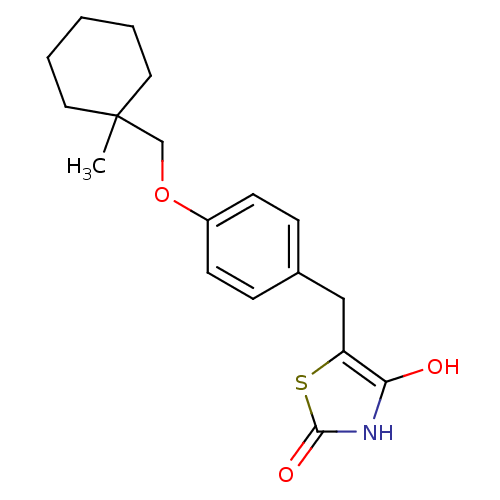 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50049243 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Activation of peroxisome proliferator activated receptor gamma measured by induction of 50% of maximum alkaline phosphatase activity, transfection as... | J Med Chem 41: 5020-36 (1999) Checked by Author Article DOI: 10.1021/jm9804127 BindingDB Entry DOI: 10.7270/Q20K2B28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50049243 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern Ohio Universities Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B after 15 mins | Bioorg Med Chem Lett 20: 5295-8 (2010) Article DOI: 10.1016/j.bmcl.2010.06.128 BindingDB Entry DOI: 10.7270/Q2S46SX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50049243 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma expressed in mouse NIH/3T3 cells assessed as receptor activation after 16 hrs by luciferase based reporter gene a... | J Nat Prod 65: 616-7 (2002) BindingDB Entry DOI: 10.7270/Q2KK9BH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD+] (Homo sapiens (Human)) | BDBM50049243 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of PGDH (unknown origin) | Bioorg Med Chem Lett 24: 630-5 (2014) Article DOI: 10.1016/j.bmcl.2013.11.081 BindingDB Entry DOI: 10.7270/Q28G8N6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD+] (Homo sapiens (Human)) | BDBM50049243 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50049243 (5-[4-(1-Methyl-cyclohexylmethoxy)-benzyl]-thiazoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro transcriptional activation of Peroxisome proliferator activated receptor gamma (PPAR) expressed in CV-1 cells | J Med Chem 39: 665-8 (1996) Article DOI: 10.1021/jm950395a BindingDB Entry DOI: 10.7270/Q2H9949G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||