

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049395 5,7,4'-Trihydroxy-6-methoxyflavone::5,7-Dihydroxy-2-(4-hydroxy-phenyl)-6-methoxy-chromen-4-one::5,7-dihydroxy-2-(4-hydroxyphenyl)-6-methoxy-4H-chromen-4-one::6-methoxy apigenin::6-methoxyapigenin::CHEMBL293776::Hispidulin::NSC-122415::TCMDC-123942::cid_5281628::dinatin
SMILES: COc1c(O)cc2oc(cc(=O)c2c1O)-c1ccc(O)cc1
InChI Key: InChIKey=IHFBPDAQLQOCBX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50049395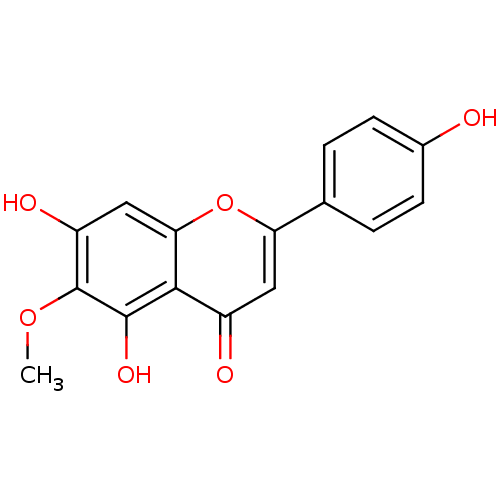 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Displacement of specific [3H]-PIA binding from adenosine A1 receptor in rat brain membranes. | J Med Chem 39: 781-8 (1996) Article DOI: 10.1021/jm950661k BindingDB Entry DOI: 10.7270/Q2M32TV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]N6-phenylisopropyladenosine binding from adenosine A1 receptor. | J Med Chem 41: 46-52 (1998) Checked by Author Article DOI: 10.1021/jm970446z BindingDB Entry DOI: 10.7270/Q2NC62QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Affinity at Adenosine A2A receptor in rat striatal membranes by [3H]- CGS 21680 displacement. | J Med Chem 39: 781-8 (1996) Article DOI: 10.1021/jm950661k BindingDB Entry DOI: 10.7270/Q2M32TV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine Receptors A2a (A2a) (Rattus norvegicus (rat)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace [3H]-CGS- 21680 binding from adenosine A2A receptor. | J Med Chem 41: 46-52 (1998) Checked by Author Article DOI: 10.1021/jm970446z BindingDB Entry DOI: 10.7270/Q2NC62QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division protein kinase 5 (Rattus norvegicus) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Inhibition of rat fetal brain CDK5 assessed as phosphorylated histone H1 levels by immuno-precipitation | J Nat Prod 67: 416-20 (2004) Article DOI: 10.1021/np034011s BindingDB Entry DOI: 10.7270/Q2ZP45WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Mus musculus) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a |
Institute of Chinese Materia Medica Curated by ChEMBL | Assay Description Agonist activity at mouse PPARgamma expressed in HEK293 cells co-expressing with Gal4 reporter vector after 24 hrs by dual-luciferase reporter assay | J Nat Prod 77: 1594-600 (2014) Article DOI: 10.1021/np500150f BindingDB Entry DOI: 10.7270/Q2D2208C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan Academia Sinica Curated by ChEMBL | Assay Description Inhibition of recombinant His6-tagged Pim-1 (unknown origin) expressed in Escherichia coli BL21 (DE3) | J Nat Prod 78: 1969-76 (2015) BindingDB Entry DOI: 10.7270/Q24B3331 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase IV (Sus scrofa (pig)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine DPP4 after 30 mins by luminescence assay | Eur J Med Chem 151: 145-157 (2018) Article DOI: 10.1016/j.ejmech.2018.03.041 BindingDB Entry DOI: 10.7270/Q2CZ39SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of Influenza A Jinan/15/90 H3N2 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Bioorg Med Chem 16: 7141-7 (2008) Article DOI: 10.1016/j.bmc.2008.06.049 BindingDB Entry DOI: 10.7270/Q2R2145D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of Influenza A Jiangsu/10/2003 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Bioorg Med Chem 16: 7141-7 (2008) Article DOI: 10.1016/j.bmc.2008.06.049 BindingDB Entry DOI: 10.7270/Q2R2145D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking Union Medical College Curated by ChEMBL | Assay Description Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Bioorg Med Chem 16: 7141-7 (2008) Article DOI: 10.1016/j.bmc.2008.06.049 BindingDB Entry DOI: 10.7270/Q2R2145D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Eur J Med Chem 45: 1724-30 (2010) Article DOI: 10.1016/j.ejmech.2010.01.005 BindingDB Entry DOI: 10.7270/Q27P926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.39E+10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires Curated by ChEMBL | Assay Description Inhibition of Influenza A PR/8/34 H1N1 virus neuraminidase activity by MUN-ANA substrate based fluorimetric assay | Eur J Med Chem 45: 1724-30 (2010) Article DOI: 10.1016/j.ejmech.2010.01.005 BindingDB Entry DOI: 10.7270/Q27P926M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroidogenic Factor 1 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | >6.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | PubChem Bioassay (2013) BindingDB Entry DOI: 10.7270/Q2ZG6QV9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| nuclear receptor subfamily 0 group B member 1 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | >834 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | PubChem Bioassay (2013) BindingDB Entry DOI: 10.7270/Q23777B0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant PIM1 (unknown origin) expressed in Escherichia coli after 60 mins by ELISA | Bioorg Med Chem 27: 677-685 (2019) Article DOI: 10.1016/j.bmc.2019.01.027 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50049395 (5,7,4'-Trihydroxy-6-methoxyflavone | 5,7-Dihydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||