

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049509 (R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl)methyl)-4-methoxyphenol::5-Bromo-2-((R)-2,2-dimethyl-6-methylene-cyclohexylmethyl)-4-methoxy-phenol::CHEMBL417729
SMILES: COc1cc(C[C@H]2C(=C)CCCC2(C)C)c(O)cc1Br
InChI Key: InChIKey=NHOGRLKDZYGUSJ-ZDUSSCGKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50049509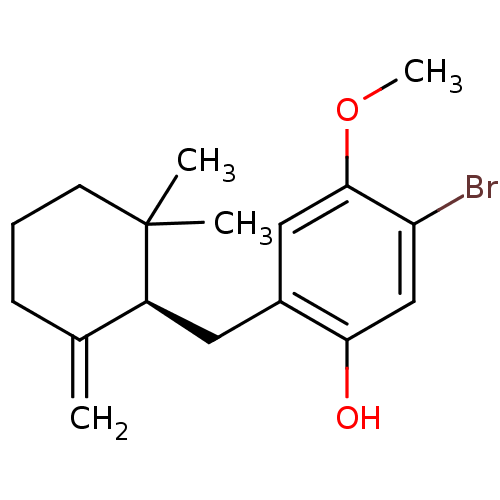 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity determined for human Progesterone receptor A isoform | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 737 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity determined against human Androgen receptor | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Displacement of [3H]DEX from human glucocorticoid receptor | J Med Chem 53: 3065-74 (2010) Article DOI: 10.1021/jm901452y BindingDB Entry DOI: 10.7270/Q2VQ33NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined for human glucocorticoid receptor(hGR). | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Androgen receptor | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Concentration required to give half-maximal inhibition against human Progesterone receptor B isoform in co-transfected CV-1 cell lines. | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human glucocorticoid receptor (hGR) | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human mineralocorticoid receptor (hMR) | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50049509 ((R)-5-bromo-2-((2,2-dimethyl-6-methylenecyclohexyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonistic potency to the human progesterone receptor measured in the T-47D alkaline phosphatase assay | J Med Chem 39: 1778-89 (1996) Article DOI: 10.1021/jm950747d BindingDB Entry DOI: 10.7270/Q2416XQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||